filmov
tv
Calculate the pH of \( 0.5 \mathrm{~L} \) of a \( 0.2 \mathrm{M} \m...

Показать описание
Calculate the pH of \( 0.5 \mathrm{~L} \) of a \( 0.2 \mathrm{M} \mathrm{NH}_{4} \mathrm{Cl}-0.2 \mathrm{M} \mathrm{NH}_{3} \) buffer before and after addition of (a) \( 0.05 \mathrm{~mole} \) of \( \mathrm{NaOH} \) and (b) \( 0.05 \) mole of \( \mathrm{HCl} \). Assume that the volume remains constant.
[Given : \( \mathrm{pK}_{\mathrm{b}} \) of \( \left.\mathrm{NH}_{3}=4.74\right] \)
[Given : \( \mathrm{pK}_{\mathrm{b}} \) of \( \left.\mathrm{NH}_{3}=4.74\right] \)
 0:13:50
0:13:50
 0:08:36
0:08:36
 0:03:32
0:03:32
 0:02:54
0:02:54
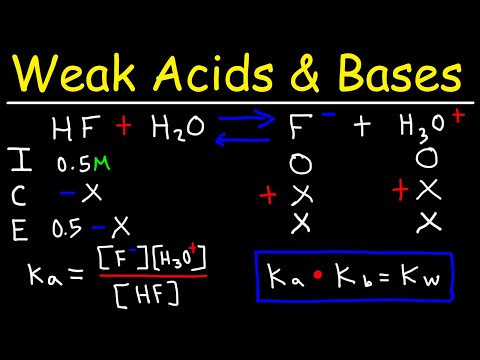 0:29:31
0:29:31
 0:04:05
0:04:05
 0:21:09
0:21:09
 0:04:37
0:04:37
 0:23:06
0:23:06
 0:09:54
0:09:54
 0:28:17
0:28:17
 0:22:56
0:22:56
 0:03:48
0:03:48
 0:03:46
0:03:46
 0:03:14
0:03:14
 0:12:37
0:12:37
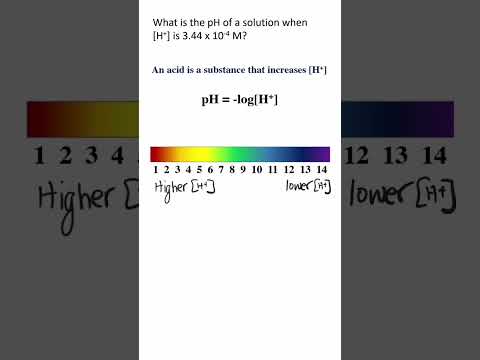 0:00:47
0:00:47
 0:11:23
0:11:23
 0:03:12
0:03:12
 0:11:26
0:11:26
 0:04:07
0:04:07
 0:03:12
0:03:12
 0:00:46
0:00:46
 0:05:45
0:05:45