filmov
tv
Mechanical Engineering Thermodynamics - Lec 23, pt 4 of 4: Example - Ideal Vapor-Compression
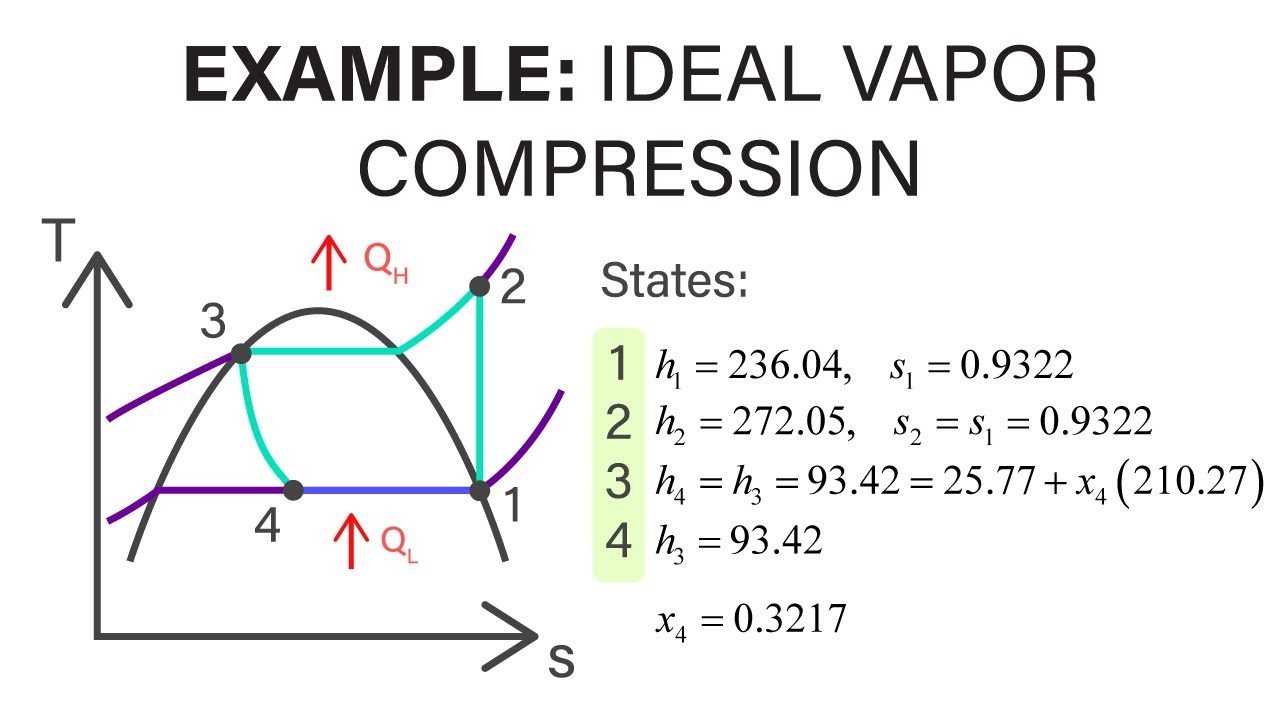
Показать описание
Problem source: Q10.14, Cengel and Boles, Thermodynamics, 3rd Edition
Mechanical Engineering Thermodynamics - Lec 11, pt 1 of 5: Exergy - Introduction
Mechanical Engineering Thermodynamics - Lec 1, pt 1 of 5: Introduction
Mechanical Engineering Thermodynamics - Lec 19, pt 1 of 5: Vapor Power Cycle Introduction
Mechanical Engineering Thermodynamics - Lec 23, pt 1 of 4: Introduction to Refrigeration Cycles
Mechanical Engineering Thermodynamics - Lec 24, pt 1 of 4: Industrial Refrigeration Applications
Mechanical Engineering Thermodynamics - Lec 7, pt 1 of 3: Reversible Process
Mechanical Engineering Thermodynamics - Lec 2, pt 4 of 5: System State / Processes
Mechanical Engineering Thermodynamics - Lec 1, pt 2 of 5: Conventional Fireplace
Mechanical Engineering Thermodynamics - Lec 19, pt 3 of 5: Rankine Cycle - Boiler
Mechanical Engineering Thermodynamics - Lec 5, pt 2 of 3: Example - First Law - Closed System
Mechanical Engineering Thermodynamics - Lec 29, pt 4 of 6: Heating and Cooling
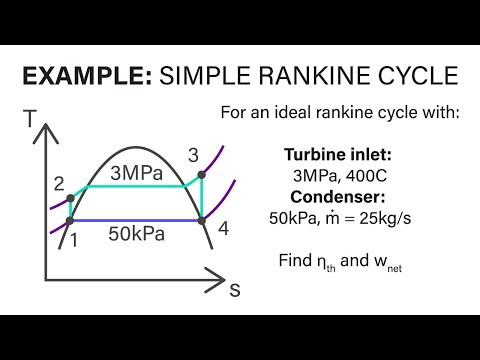
Mechanical Engineering Thermodynamics - Lec 21, pt 1 of 5: Example - Simple Rankine Cycle
Mechanical Engineering Thermodynamics - Lec 12, pt 1 of 4: Exergy - Internal Energy
Mechanical Engineering Thermodynamics - Lec 20, pt 1 of 7: Actual Rankine Cycle
Mechanical Engineering Thermodynamics - Lec 29, pt 2 of 6: Air-Conditioning Processes Introduction
Mechanical Engineering Thermodynamics - Lec 6, pt 2 of 4: First Law and the Wake of a Baseball
Mechanical Engineering Thermodynamics - Lec 2, pt 1 of 5: Terminology / Equations
Mechanical Engineering Thermodynamics - Lec 33, pt 3 of 3: Example - Reacting Systems pt ii
Mechanical Engineering Thermodynamics - Lec 22, pt 1 of 3: Rankine with Cogeneration
Mechanical Engineering Thermodynamics - Lec 9, pt 3 of 5: Isentropic Efficiencies
Mechanical Engineering Thermodynamics - Lec 4, pt 1 of 3: Heat and Work
Mechanical Engineering Thermodynamics - Lec 25, pt 1 of 4: Gas Refrigeration Cycles
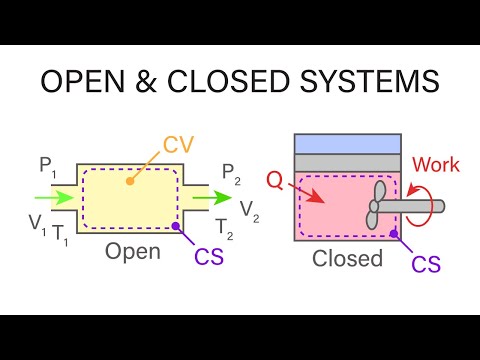
Mechanical Engineering Thermodynamics - Lec 2, pt 2 of 5: Closed / Open Systems
Mechanical Engineering Thermodynamics - Lec 34, pt 1 of 4: Adiabatic Flame Temperature
Комментарии
 0:05:57
0:05:57
 0:12:36
0:12:36
 0:06:40
0:06:40
 0:10:38
0:10:38
 0:07:03
0:07:03
 0:06:11
0:06:11
 0:12:54
0:12:54
 0:06:02
0:06:02
 0:11:55
0:11:55
 0:14:41
0:14:41
 0:06:59
0:06:59
 0:14:43
0:14:43
 0:13:55
0:13:55
 0:10:02
0:10:02
 0:03:53
0:03:53
 0:12:23
0:12:23
 0:07:50
0:07:50
 0:08:00
0:08:00
 0:07:18
0:07:18
 0:12:43
0:12:43
 0:13:48
0:13:48
 0:07:14
0:07:14
 0:07:39
0:07:39
 0:10:57
0:10:57