filmov
tv
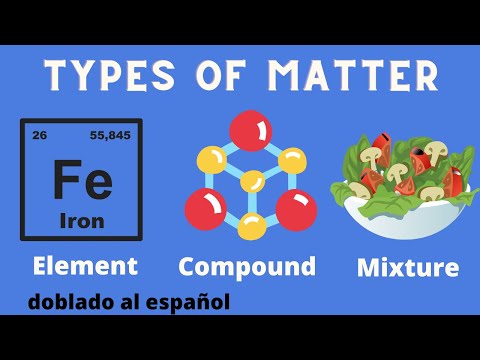
Difference between mixture and compound?

Показать описание
In this video, I will explain the difference between mixture and compound. A mixture is a combination of two or more substances that are not chemically bonded, while a compound is a substance that is made up of two or more elements that are chemically bonded. I will also show you some examples of mixtures and compounds, and how to separate them using different methods.
A mixture can have a variable composition and properties, depending on the amount and type of substances that are mixed together. For example, salt and water form a mixture that can have different concentrations of salt, and different boiling and freezing points. A mixture can be separated by physical methods, such as filtration or evaporation, that do not change the identity of the substances. For example, you can separate salt and water by boiling the water and collecting the salt crystals.
There are two main types of mixtures: homogeneous and heterogeneous. Homogeneous mixtures have a uniform composition and appearance throughout, while heterogeneous mixtures have different properties and compositions in different parts. Homogeneous mixtures are also called solutions. A solution is a mixture of a solute and a solvent. A solute is the substance that dissolves in the solvent, and a solvent is the substance that dissolves the solute. For example, in a salt-water solution, salt is the solute and water is the solvent. Solutions can be solid, liquid, or gas. For example, air is a solution of different gases, such as oxygen, nitrogen, and carbon dioxide. Heterogeneous mixtures can be further classified into colloids and suspensions. A colloid is a mixture of very small particles of one substance dispersed in another substance. The particles are too small to be seen by the naked eye, but they are large enough to scatter light. For example, milk is a colloid of fat particles in water. A suspension is a mixture of larger particles of one substance suspended in another substance. The particles are visible and tend to settle down when left undisturbed. For example, sand and water form a suspension that can be separated by filtration.
A compound is a substance that is made up of two or more elements that are chemically bonded. This means that the different elements of a compound are joined together by sharing or transferring electrons. For example, water is a compound of hydrogen and oxygen that are bonded by sharing electrons. Compounds have different properties from their constituent elements, because the elements lose their individual characteristics when they form a compound. For example, hydrogen and oxygen are both gases, but water is a liquid. Compounds have a fixed ratio of elements that cannot be changed by physical methods. For example, water always has two atoms of hydrogen and one atom of oxygen in each molecule. Compounds can only be separated into simpler substances by chemical methods, such as electrolysis or decomposition, that change the identity of the substances. For example, water can be separated into hydrogen and oxygen by passing an electric current through it.
The main difference between mixture and compound is that a mixture is a physical combination of substances, while a compound is a chemical combination of elements. A mixture can have a variable composition and properties, while a compound has a fixed composition and properties. A mixture can be separated by physical methods, while a compound can be separated by chemical methods.
I hope this video helps you understand the difference between mixture and compound better. If you have any questions or comments, please leave them below. Thank you for watching and have a great day! 😊
A mixture can have a variable composition and properties, depending on the amount and type of substances that are mixed together. For example, salt and water form a mixture that can have different concentrations of salt, and different boiling and freezing points. A mixture can be separated by physical methods, such as filtration or evaporation, that do not change the identity of the substances. For example, you can separate salt and water by boiling the water and collecting the salt crystals.
There are two main types of mixtures: homogeneous and heterogeneous. Homogeneous mixtures have a uniform composition and appearance throughout, while heterogeneous mixtures have different properties and compositions in different parts. Homogeneous mixtures are also called solutions. A solution is a mixture of a solute and a solvent. A solute is the substance that dissolves in the solvent, and a solvent is the substance that dissolves the solute. For example, in a salt-water solution, salt is the solute and water is the solvent. Solutions can be solid, liquid, or gas. For example, air is a solution of different gases, such as oxygen, nitrogen, and carbon dioxide. Heterogeneous mixtures can be further classified into colloids and suspensions. A colloid is a mixture of very small particles of one substance dispersed in another substance. The particles are too small to be seen by the naked eye, but they are large enough to scatter light. For example, milk is a colloid of fat particles in water. A suspension is a mixture of larger particles of one substance suspended in another substance. The particles are visible and tend to settle down when left undisturbed. For example, sand and water form a suspension that can be separated by filtration.
A compound is a substance that is made up of two or more elements that are chemically bonded. This means that the different elements of a compound are joined together by sharing or transferring electrons. For example, water is a compound of hydrogen and oxygen that are bonded by sharing electrons. Compounds have different properties from their constituent elements, because the elements lose their individual characteristics when they form a compound. For example, hydrogen and oxygen are both gases, but water is a liquid. Compounds have a fixed ratio of elements that cannot be changed by physical methods. For example, water always has two atoms of hydrogen and one atom of oxygen in each molecule. Compounds can only be separated into simpler substances by chemical methods, such as electrolysis or decomposition, that change the identity of the substances. For example, water can be separated into hydrogen and oxygen by passing an electric current through it.
The main difference between mixture and compound is that a mixture is a physical combination of substances, while a compound is a chemical combination of elements. A mixture can have a variable composition and properties, while a compound has a fixed composition and properties. A mixture can be separated by physical methods, while a compound can be separated by chemical methods.
I hope this video helps you understand the difference between mixture and compound better. If you have any questions or comments, please leave them below. Thank you for watching and have a great day! 😊
 0:04:16
0:04:16
 0:06:26
0:06:26
 0:06:06
0:06:06
 0:04:15
0:04:15
 0:05:16
0:05:16
 0:07:34
0:07:34
 0:03:58
0:03:58
 0:03:17
0:03:17
 1:00:54
1:00:54
 0:07:11
0:07:11
 0:05:06
0:05:06
 0:08:15
0:08:15
 0:09:29
0:09:29
 0:01:30
0:01:30
 0:08:21
0:08:21
 0:00:15
0:00:15
 0:00:19
0:00:19
 0:02:39
0:02:39
 0:00:09
0:00:09
 0:07:20
0:07:20
 0:06:12
0:06:12
 0:06:26
0:06:26
 0:00:58
0:00:58
 0:04:57
0:04:57