filmov
tv
Understanding the De Broglie Wave Equation in Quantum Mechanics

Показать описание
The Davisson-Germer experiment was a fundamental experiment in quantum mechanics that provided direct evidence for the wave nature of electrons, supporting de Broglie's hypothesis of matter waves.
Overview:
Conducted by: Clinton Davisson and Lester Germer in 1927.
Objective: To study the scattering of electrons from a crystalline nickel target.
Significance: It confirmed the wave-particle duality of electrons by showing that electrons can exhibit diffraction patterns, which are characteristic of waves.
Experimental Setup:
Electron Gun: A beam of electrons was accelerated by applying a potential difference and directed at a nickel crystal target.
Nickel Crystal: The electrons were scattered off the atoms of the nickel crystal.
Detector: A movable detector measured the intensity of the scattered electrons at various angles.
Key Findings:
When the electrons were scattered from the surface of the nickel crystal, they produced a diffraction pattern similar to X-ray diffraction patterns from crystals.
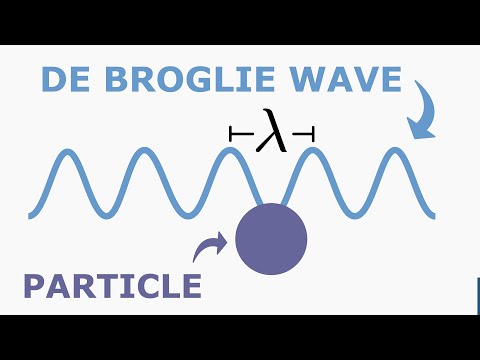
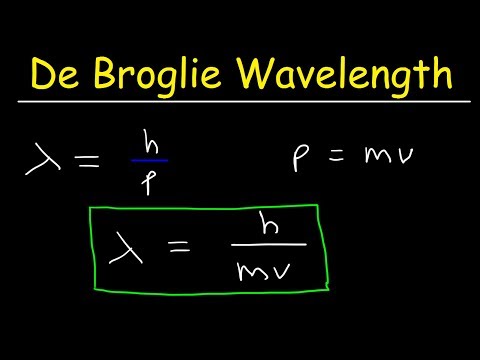
The wavelength of the electrons, calculated using Bragg's law, matched the de Broglie wavelength, λ=hpλ=ph, where hh is Planck's constant and pp is the momentum of the electron.
Conclusion:
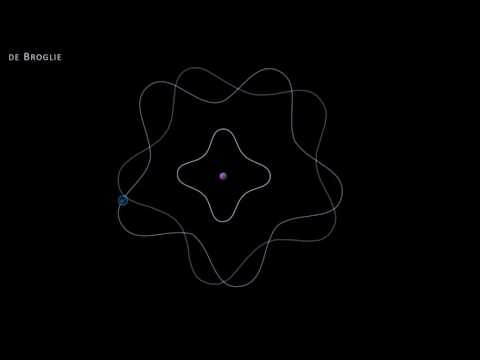
The Davisson-Germer experiment provided crucial experimental verification of de Broglie's theory that particles such as electrons have wave-like properties, thus supporting the quantum mechanical description of matter.
This experiment laid the groundwork for the development of quantum mechanics and played a key role in the formulation of the wave-particle duality concept.
#chemistry #education #wavemechanics #debroglie #atom
Overview:
Conducted by: Clinton Davisson and Lester Germer in 1927.
Objective: To study the scattering of electrons from a crystalline nickel target.
Significance: It confirmed the wave-particle duality of electrons by showing that electrons can exhibit diffraction patterns, which are characteristic of waves.
Experimental Setup:
Electron Gun: A beam of electrons was accelerated by applying a potential difference and directed at a nickel crystal target.
Nickel Crystal: The electrons were scattered off the atoms of the nickel crystal.
Detector: A movable detector measured the intensity of the scattered electrons at various angles.
Key Findings:
When the electrons were scattered from the surface of the nickel crystal, they produced a diffraction pattern similar to X-ray diffraction patterns from crystals.
The wavelength of the electrons, calculated using Bragg's law, matched the de Broglie wavelength, λ=hpλ=ph, where hh is Planck's constant and pp is the momentum of the electron.
Conclusion:
The Davisson-Germer experiment provided crucial experimental verification of de Broglie's theory that particles such as electrons have wave-like properties, thus supporting the quantum mechanical description of matter.
This experiment laid the groundwork for the development of quantum mechanics and played a key role in the formulation of the wave-particle duality concept.
#chemistry #education #wavemechanics #debroglie #atom
 0:11:20
0:11:20
 0:05:15
0:05:15
 0:09:05
0:09:05
 0:19:00
0:19:00
 0:04:29
0:04:29
 0:11:21
0:11:21
 0:07:35
0:07:35
 0:10:37
0:10:37
 0:00:56
0:00:56
 0:02:06
0:02:06
 0:11:53
0:11:53
 0:08:12
0:08:12
 0:01:43
0:01:43
 0:08:41
0:08:41
 0:21:29
0:21:29
 0:10:11
0:10:11
 0:20:24
0:20:24
 0:09:34
0:09:34
 0:07:01
0:07:01
 0:06:56
0:06:56
 0:01:51
0:01:51
 0:11:20
0:11:20
 0:03:41
0:03:41
 0:10:26
0:10:26