filmov
tv
R2.3.5 What is the Difference Between Kc and Q? [SL IB Chemistry]

Показать описание
KC involves concentrations of the reactants and products at equilibrium, but Q uses the concentrations on their way to equilibrium.
Composition of relations | MISTAKE - explained RoS instead of SoR and vice versa | otherwise correct
Memorization Trick for Graphing Functions Part 1 | Algebra Math Hack #shorts #math #school
New Trick Imporove Aim while Scoping ✅ #pubgmobile #bgmi #ruby__yt
NEWYES Calculator VS Casio calculator
series 2^n/(3^n+5),convergent or divergent,Direct comparison test#shorts
Solve The Last 3 Pieces (in 1 SECOND)
Compare the ratios: 5:6 and 3:4 | 6 | RATIO AND PROPORTION | MATHS | ICSE | Doubtnut
15 FPS VS 30 FPS VS 60 FPS GTA 5 #shorts
Does a $5 AM5 Contact Frame Actually Make a Difference?
AMD Ryzen 5 5600 vs 5600X vs 5600G! What's The Difference?🤷♂️ #gamingpc #ryzen5 #5600 #5600x #...
Ryzen 5 5600X tops cinebench in single core performance!!!
POV: You Only Solved 3 Sides 💀 #shorts
What is ITR 1 2 3 4 5 6 | ITR 1 2 3 4 5 6 meaning | How to choose itr forms | ITR kya hota hai | itr
Infinite chocolate trick explained
5 key differences between Nike React 4 and Nike React 3! #nikereact #react
The ACTUAL Difference Between Intel and AMD
My Weirdest Rubik’s cubes #shorts
Japanese vs. German Chef Knives
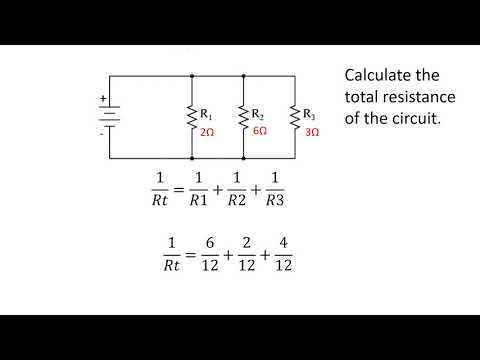
Calculating resistance in parallel
This 3 second Rubik’s cube solve is INSANE 😯
How to brush using an electric toothbrush. #brush #brushing #dentist
What is a Core i3, Core i5, or Core i7 as Fast As Possible
3 Tips To Defend Like A Pro Player In FIFA 23
MY TOP 3 MARATHON SHOES
Комментарии
 0:03:20
0:03:20
 0:00:15
0:00:15
 0:00:31
0:00:31
 0:00:14
0:00:14
 0:00:55
0:00:55
 0:00:31
0:00:31
 0:01:53
0:01:53
 0:00:17
0:00:17
 0:03:07
0:03:07
 0:00:43
0:00:43
 0:00:15
0:00:15
 0:00:09
0:00:09
 0:06:38
0:06:38
 0:00:48
0:00:48
 0:00:35
0:00:35
 0:05:27
0:05:27
 0:00:19
0:00:19
 0:01:01
0:01:01
 0:03:35
0:03:35
 0:00:35
0:00:35
 0:00:12
0:00:12
 0:04:32
0:04:32
 0:00:35
0:00:35
 0:00:21
0:00:21