filmov
tv
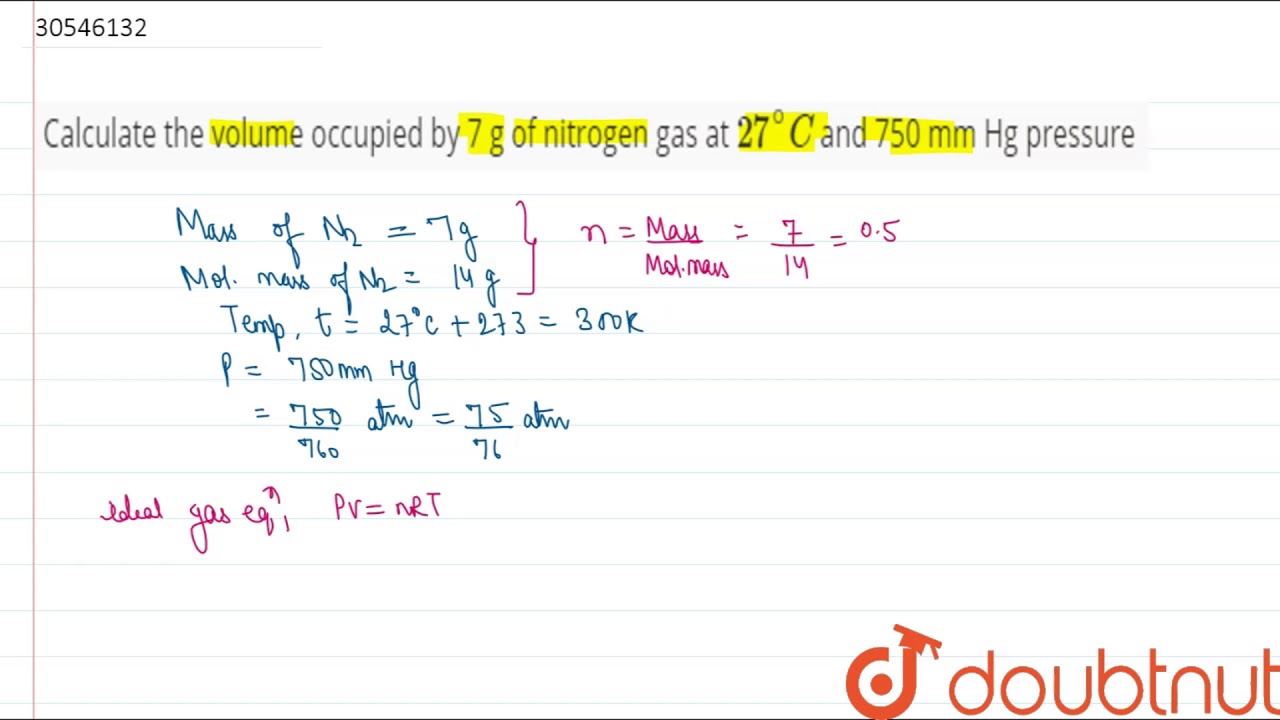
Calculate the volume occupied by 7 g of nitrogen gas at `27^(@)C` and 750 mm Hg pressure
Показать описание
Calculate the volume occupied by 7 g of nitrogen gas at `27^(@)C` and 750 mm Hg pressure
15.3 Calculate the volume occupied by a gram-mole of a gas at 0 ⁰C and a pressure of 1.0 atmosphere...
Calculate the volume occupied by 0.8 g of methane at STP. W
Calculate the volume occupied by 5.0 g of acetylene gas at 50^(@)C and 740 mm pressure. | 11 | S...
Calculate the volume occupied by `5.0 g` of acetylene gas at `50^(@)C` and `740 mm` pressure.
How to calculate volume at STP
Calculate the volume occupied by 4.0245 × 10^23 molecules of O_2 at 27^∘C having pressure of 700 ......
Calculate the volume occupied by 4 mole of an ideal gas at `2.5 xx 10^(5) Nm^(-2)
Calculate the volume occupied by 7 grams of nitrogen gas (molar mass = 28 g) | 10 | MOLE CONCEPT...
Volume
Calculate the volume occupied by 0 845 mol of nitrogen gas at a pressure of 1 37 atm and temperature
Calculate the volume occupied at S.T.P. by 2 moles of carbon dioxide. (C = 12, O = 16) | 10 | M...
Calculate the volume occupied by `5.0 g` of acetylene gas at `50^()C` and `740 mm` pressure.
CALCULATE THE VOLUME AT RTP
Calculate the volume occupied by `5.0 g` of acetylene gas at `50^()C` and `740 mm` pressure.
Calculate the volume occupied by 11.7 moles of carbon monoxide gas at STP.
9.43b | Calculate the volume occupied by 10.0 g of each of these compounds at STP: CH3CH2F(g)
Calculate the volume occupied by `4.045 xx 10^(2)` molecules of oxygen at `27^(@)C`
How to calculate volume of a gas using molar gas volume 24dm^3/mole
The volume occupied by 35.5g of `CI_(2)` at S.T.P.
MCQ The volume occupied by 2.8 grams of 𝑁 2 at STP is
Calculate the volume occupied at `27^(@)C` and 2 bar pressure of a gas evolved
Calculate the volume occupied by `5.0 g` of acetylene gas at `50^()C` and `740 mm` pressure.
the volume occupied by 1.4 gram of nitrogen at STP
Q11 Calculate the Volume occupied by 5.25g of Nitrogen at 26 degree celcius and 74.2cm of pressure
Комментарии
 0:01:06
0:01:06
 0:02:35
0:02:35
 0:03:54
0:03:54
 0:02:55
0:02:55
 0:01:58
0:01:58
 0:01:23
0:01:23
 0:02:18
0:02:18
 0:03:08
0:03:08
 0:30:37
0:30:37
 0:01:47
0:01:47
 0:01:33
0:01:33
 0:03:53
0:03:53
 0:00:59
0:00:59
 0:04:48
0:04:48
 0:00:33
0:00:33
 0:06:05
0:06:05
 0:02:54
0:02:54
 0:05:19
0:05:19
 0:03:17
0:03:17
 0:03:12
0:03:12
 0:01:46
0:01:46
 0:02:54
0:02:54
 0:02:39
0:02:39
 0:01:21
0:01:21