filmov
tv
Trick To Write Electronic Configuration Of Elements Without Moeller Chart : JEE MAINS, NEET, EAMCET

Показать описание
Today I'll share a wonderful trick to remember the order of electronic configuration of elements without the help of moeller chart in the least time and space.
WHAT IS AUFBAU PRINCIPLE :
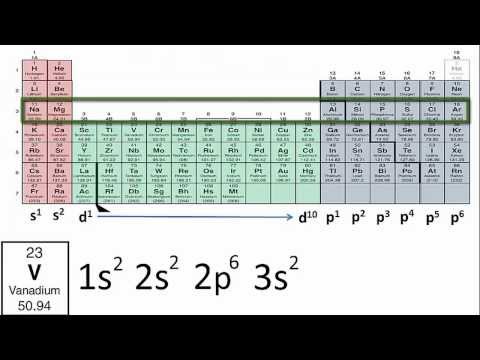
The Aufbau principle dictates the manner in which electrons are filled in the atomic orbitals of an atom in its ground state. It states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. According to the Aufbau principle, the available atomic orbitals with the lowest energy levels are occupied before those with higher energy levels.
The word ‘Aufbau’ has German roots and can be roughly translated as ‘construct’ or ‘build up’. A diagram illustrating the order in which atomic orbitals are filled is provided below. Here, ‘n’ refers to the principal quantum number and ‘l’ is the azimuthal quantum number
The Aufbau principle can be used to understand the location of electrons in an atom and their corresponding energy levels. For example, carbon has 6 electrons and its electronic configuration is 1s²2s²2p².
It is important to note that each orbital can hold a maximum of two electrons (as per the Pauli exclusion principle). Also, the manner in which electrons are filled into orbitals in a single subshell must follow Hund’s rule, i.e. every orbital in a given subshell must be singly occupied by electrons before any two electrons pair up in an orbital.
Salient Features of the Aufbau Principle
According to the Aufbau principle, electrons first occupy those orbitals whose energy is the lowest. This implies that the electrons enter the orbitals having higher energies only when orbitals with lower energies have been completely filled.
The order in which the energy of orbitals increases can be determined with the help of the (n+l) rule, where the sum of the principal and azimuthal quantum numbers determines the energy level of the orbital.
Lower (n+l) values correspond to lower orbital energies. If two orbitals share equal (n+l) values, the orbital with the lower n value is said to have lower energy associated with it.
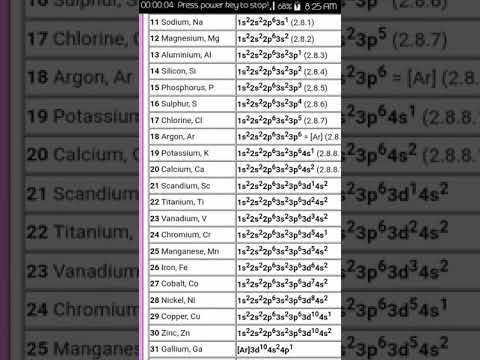
The order in which the orbitals are filled with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on
Electronic Configuration using the Aufbau Principle
Writing the Electron Configuration of Sulphur
The atomic number of sulphur is 16, implying that it holds a total of 16 electrons.
As per the Aufbau principle, two of these electrons are present in the 1s subshell, eight of them are present in the 2s and 2p subshell, and the remaining are distributed into the 3s and 3p subshells.
Therefore, the electron configuration of sulphur can be written as 1s²2s²2p⁶3s²3p⁴.
Writing the Electron Configuration of Nitrogen
The element nitrogen has 7 electrons (since its atomic number is 7).
The electrons are filled into the 1s, 2s, and 2p orbitals.
The electron configuration of nitrogen can be written as 1s²2s²2p³
If you like the video, please share this video with your friends and family.
And leave a comment and like the video and don't forget to subscribe.
#electronic #configuration #moeller #chart #diagram #chemistry #10thclass #11thclass #moeller #chart #diagram #intermediate #cbse #stateboard #hindi #english #telugu #ganni #tricks #physics #aufbau #principle
#hundsrule #aufbauprinciple #paulisexclusionprinciple
Tags :
how to draw a Moeller chart
how to use Moeller diagram in English
how to write the electronic configuration of elements easily
10th class chemistry tricks
11th class chemistry tricks
intermediate chemistry tricks
iit jee tricks
jee mains tricks
jee mains chemistry tricks
ap eamcet tricks
ts eamcet tricks
ap eamcet chemistry tricks
ts eamcet chemistry tricks
neet chemistry tricks
WHAT IS AUFBAU PRINCIPLE :
The Aufbau principle dictates the manner in which electrons are filled in the atomic orbitals of an atom in its ground state. It states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. According to the Aufbau principle, the available atomic orbitals with the lowest energy levels are occupied before those with higher energy levels.
The word ‘Aufbau’ has German roots and can be roughly translated as ‘construct’ or ‘build up’. A diagram illustrating the order in which atomic orbitals are filled is provided below. Here, ‘n’ refers to the principal quantum number and ‘l’ is the azimuthal quantum number
The Aufbau principle can be used to understand the location of electrons in an atom and their corresponding energy levels. For example, carbon has 6 electrons and its electronic configuration is 1s²2s²2p².
It is important to note that each orbital can hold a maximum of two electrons (as per the Pauli exclusion principle). Also, the manner in which electrons are filled into orbitals in a single subshell must follow Hund’s rule, i.e. every orbital in a given subshell must be singly occupied by electrons before any two electrons pair up in an orbital.
Salient Features of the Aufbau Principle
According to the Aufbau principle, electrons first occupy those orbitals whose energy is the lowest. This implies that the electrons enter the orbitals having higher energies only when orbitals with lower energies have been completely filled.
The order in which the energy of orbitals increases can be determined with the help of the (n+l) rule, where the sum of the principal and azimuthal quantum numbers determines the energy level of the orbital.
Lower (n+l) values correspond to lower orbital energies. If two orbitals share equal (n+l) values, the orbital with the lower n value is said to have lower energy associated with it.
The order in which the orbitals are filled with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on
Electronic Configuration using the Aufbau Principle
Writing the Electron Configuration of Sulphur
The atomic number of sulphur is 16, implying that it holds a total of 16 electrons.
As per the Aufbau principle, two of these electrons are present in the 1s subshell, eight of them are present in the 2s and 2p subshell, and the remaining are distributed into the 3s and 3p subshells.
Therefore, the electron configuration of sulphur can be written as 1s²2s²2p⁶3s²3p⁴.
Writing the Electron Configuration of Nitrogen
The element nitrogen has 7 electrons (since its atomic number is 7).
The electrons are filled into the 1s, 2s, and 2p orbitals.
The electron configuration of nitrogen can be written as 1s²2s²2p³
If you like the video, please share this video with your friends and family.
And leave a comment and like the video and don't forget to subscribe.
#electronic #configuration #moeller #chart #diagram #chemistry #10thclass #11thclass #moeller #chart #diagram #intermediate #cbse #stateboard #hindi #english #telugu #ganni #tricks #physics #aufbau #principle
#hundsrule #aufbauprinciple #paulisexclusionprinciple
Tags :
how to draw a Moeller chart
how to use Moeller diagram in English
how to write the electronic configuration of elements easily
10th class chemistry tricks
11th class chemistry tricks
intermediate chemistry tricks
iit jee tricks
jee mains tricks
jee mains chemistry tricks
ap eamcet tricks
ts eamcet tricks
ap eamcet chemistry tricks
ts eamcet chemistry tricks
neet chemistry tricks
Комментарии
 0:04:36
0:04:36
 0:07:23
0:07:23
 0:10:19
0:10:19
 0:04:52
0:04:52
 0:09:05
0:09:05
 0:07:48
0:07:48
 0:00:50
0:00:50
 0:00:29
0:00:29
 0:00:57
0:00:57
 0:04:14
0:04:14
 0:06:29
0:06:29
 0:09:23
0:09:23
 0:08:42
0:08:42
 0:17:04
0:17:04
 0:00:12
0:00:12
 0:13:36
0:13:36
 0:18:05
0:18:05
 0:00:54
0:00:54
 0:00:56
0:00:56
 0:00:32
0:00:32
 0:17:15
0:17:15
 0:12:13
0:12:13
 0:05:26
0:05:26
 0:07:39
0:07:39