filmov
tv
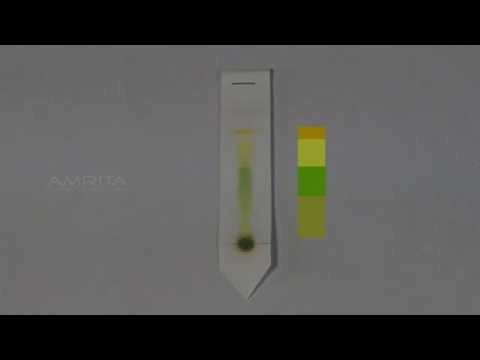
Paper Chromatography

Показать описание
LAB MANUAL
Experiment: Separation of Plant Pigments Using Paper Chromatography
Objective
To separate and identify the various pigments present in plant leaves using paper chromatography.
Theory
Paper chromatography is a technique used to separate and identify mixtures that are or can be colored, especially pigments. It operates on the principle of partition chromatography, where substances are distributed between a stationary phase and a mobile phase. In this method:
• Stationary Phase: The water molecules bound to the cellulose fibers of the chromatography
paper.
• Mobile Phase: A suitable solvent or solvent mixture that moves through the stationary phase, carrying the components of the mixture with it.
Different compounds in the mixture travel at different rates due to differences in their solubility in the mobile phase and their affinity to the stationary phase. This results in the separation of the components on the paper.
Materials Required
• Fresh green leaves (e.g., spinach / )
• Mortar and pestle
• Acetone or ethanol (as extraction solvent)
• Whatman No. 1 filter paper strips
• Glass jar or column
• Petroleum ether and acetone mixture (9:1 ratio) as the developing solvent
• Capillary tubes
• Pencil and ruler
• Scissors
• Beaker
• Measuring cylinder
• Distilled water
Procedure
• Preparation of Pigment Extract:
• Collect fresh green leaves and cut them into small pieces.
• Grind the leaf pieces in a mortar with a small amount of acetone to extract the pigments.
• Filter the extract using filter paper to obtain a clear solution.
• Preparation of Chromatography Paper:
• Take a strip of Whatman No. 1 filter paper
• Draw a pencil line approximately 1 cm from one end of the paper. This is the origin line.
• Using a capillary tube or fine brush, apply a small spot of the pigment extract onto the center of the origin line.
• Allow the spot to dry and repeat the application 2–3 times to concentrate the pigment at the spot.
• Development of Chromatogram:
• Prepare the developing solvent by mixing petroleum ether and acetone in a 9:1 ratio.
• Pour the solvent mixture into the chromatography chamber to a depth of about 0.5 cm.
• Suspend the chromatography paper in the chamber so that the bottom edge is immersed in the solvent, but the pigment spot remains above the solvent level.
• Ensure the chamber is sealed to prevent solvent evaporation.
• Allow the solvent to rise up the paper by capillary action until it is about 1 cm from the top edge.
• Remove the paper and immediately mark the solvent front with a pencil.
• Allow the paper to dry in a well-ventilated area or in hot air oven
• Observation and Analysis:
• Observe the separated pigment bands on the chromatography paper.
• Measure the distance traveled by each pigment band from the origin line.
• Measure the distance traveled by the solvent front from the origin line.
Calculations
Calculate the Retention factor (Rf) for each pigment using the formula:
Rf = {Distance traveled by the pigment}={Distance traveled by the solvent }
Record the Rf values for each pigment observed.
Expected Results
You may observe the following pigments separated on the chromatography paper:
• Chlorophyll a: Blue-green band
• Chlorophyll b: Yellow-green band
• Xanthophylls: Yellow band
• Carotenes: Orange band
Each pigment will have a characteristic Rf value under the given experimental conditions.
Precautions
• Use a pencil, not a pen, to mark the chromatography paper, as ink may interfere with the results.
• Ensure the pigment spot is small and concentrated for better separation.
• Do not allow the pigment spot to be submerged in the solvent.
• Handle solvents with care, using gloves and working in a well-ventilated area.
• Avoid touching the chromatography paper with bare hands to prevent contamination.
Experiment: Separation of Plant Pigments Using Paper Chromatography
Objective
To separate and identify the various pigments present in plant leaves using paper chromatography.
Theory
Paper chromatography is a technique used to separate and identify mixtures that are or can be colored, especially pigments. It operates on the principle of partition chromatography, where substances are distributed between a stationary phase and a mobile phase. In this method:
• Stationary Phase: The water molecules bound to the cellulose fibers of the chromatography
paper.
• Mobile Phase: A suitable solvent or solvent mixture that moves through the stationary phase, carrying the components of the mixture with it.
Different compounds in the mixture travel at different rates due to differences in their solubility in the mobile phase and their affinity to the stationary phase. This results in the separation of the components on the paper.
Materials Required
• Fresh green leaves (e.g., spinach / )
• Mortar and pestle
• Acetone or ethanol (as extraction solvent)
• Whatman No. 1 filter paper strips
• Glass jar or column
• Petroleum ether and acetone mixture (9:1 ratio) as the developing solvent
• Capillary tubes
• Pencil and ruler
• Scissors
• Beaker
• Measuring cylinder
• Distilled water
Procedure
• Preparation of Pigment Extract:
• Collect fresh green leaves and cut them into small pieces.
• Grind the leaf pieces in a mortar with a small amount of acetone to extract the pigments.
• Filter the extract using filter paper to obtain a clear solution.
• Preparation of Chromatography Paper:
• Take a strip of Whatman No. 1 filter paper
• Draw a pencil line approximately 1 cm from one end of the paper. This is the origin line.
• Using a capillary tube or fine brush, apply a small spot of the pigment extract onto the center of the origin line.
• Allow the spot to dry and repeat the application 2–3 times to concentrate the pigment at the spot.
• Development of Chromatogram:
• Prepare the developing solvent by mixing petroleum ether and acetone in a 9:1 ratio.
• Pour the solvent mixture into the chromatography chamber to a depth of about 0.5 cm.
• Suspend the chromatography paper in the chamber so that the bottom edge is immersed in the solvent, but the pigment spot remains above the solvent level.
• Ensure the chamber is sealed to prevent solvent evaporation.
• Allow the solvent to rise up the paper by capillary action until it is about 1 cm from the top edge.
• Remove the paper and immediately mark the solvent front with a pencil.
• Allow the paper to dry in a well-ventilated area or in hot air oven
• Observation and Analysis:
• Observe the separated pigment bands on the chromatography paper.
• Measure the distance traveled by each pigment band from the origin line.
• Measure the distance traveled by the solvent front from the origin line.
Calculations
Calculate the Retention factor (Rf) for each pigment using the formula:
Rf = {Distance traveled by the pigment}={Distance traveled by the solvent }
Record the Rf values for each pigment observed.
Expected Results
You may observe the following pigments separated on the chromatography paper:
• Chlorophyll a: Blue-green band
• Chlorophyll b: Yellow-green band
• Xanthophylls: Yellow band
• Carotenes: Orange band
Each pigment will have a characteristic Rf value under the given experimental conditions.
Precautions
• Use a pencil, not a pen, to mark the chromatography paper, as ink may interfere with the results.
• Ensure the pigment spot is small and concentrated for better separation.
• Do not allow the pigment spot to be submerged in the solvent.
• Handle solvents with care, using gloves and working in a well-ventilated area.
• Avoid touching the chromatography paper with bare hands to prevent contamination.
 0:06:33
0:06:33
 0:00:27
0:00:27
 0:02:50
0:02:50
 0:01:00
0:01:00
 0:04:02
0:04:02
 0:03:57
0:03:57
 0:11:40
0:11:40
 0:17:48
0:17:48
 0:04:28
0:04:28
 0:00:55
0:00:55
 0:00:28
0:00:28
 0:05:38
0:05:38
 0:03:49
0:03:49
 0:10:17
0:10:17
 0:00:29
0:00:29
 0:13:04
0:13:04
 0:06:39
0:06:39
 0:11:36
0:11:36
 0:01:00
0:01:00
 0:00:27
0:00:27
 0:11:35
0:11:35
 0:07:41
0:07:41
 0:03:50
0:03:50
 0:02:59
0:02:59