filmov
tv
Calculate Equilibrium Concentration of a Weak Acid from Kₐ 001

Показать описание
What is the molar concentration of hypobromous acid if the equilibrium concentration of hydronium ion is 0.401 M and the molar concentration of the conjugate base is 0.125 M. (Kₐ = 2.3 x 10⁻⁹)
HBrO (aq) + H₂O (l) ⇌ BrO⁻ (aq) + H₃O⁺ (aq)
HBrO (aq) + H₂O (l) ⇌ BrO⁻ (aq) + H₃O⁺ (aq)
Worked example: Calculating equilibrium concentrations from initial concentrations | Khan Academy
Practice Problem: Calculating Equilibrium Concentrations
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Using RICE to calculate equilibrium concentrations
Equilibrium Concentrations
Calculating Concentration - Basic Equilibrium
Equilibrium Equations: Crash Course Chemistry #29
How to Calculate Equilibrium Concentrations from the Equilibrium Constant? | Example!
Crack JEE 2025 | JEE Mantra Series 2024 | Chemistry Recap Video Lecture - 7 | JEE Main Preparation
Calculating Equilibrium Concentrations: Example 1
Calculating Equilibrium Concentrations: Example 5
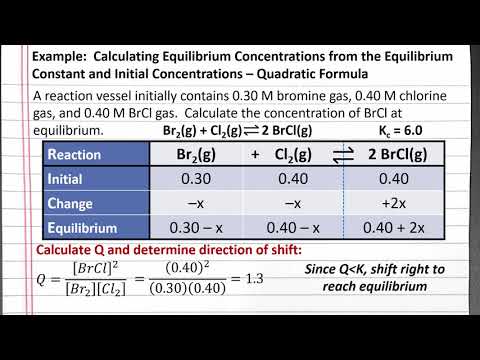
CHEM 201: Calculating Equilibrium Concentrations from K and Initial Values – Quadratic Formula
Calculate one equilibrium concentration using Kc and other concentrations
Find the equilibrium concentration using the 5% approximation rule
Chemical Equilibrium Problem 02 | Determining Equilibrium Concentration | Using Quadratic Equations
Calculating Kp or Kc given an equilibrium concentration
Equilibrium Constant Grade 12: Exam
Chemical Equilibria and Reaction Quotients
How to calculate the equilibrium concentration of a reactant or product
Calculate Keq from Equilibrium Concentrations
ICE Tables made EASY!
Equilibrium Calculations 2: Equilibrium Concentrations
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
ICE Table Practice Problems - Initial Concentration, Equilibrium Concentration, Kc (Part 1)
Комментарии
 0:05:29
0:05:29
 0:03:02
0:03:02
 0:53:22
0:53:22
 0:10:13
0:10:13
 0:03:55
0:03:55
 0:03:38
0:03:38
 0:09:29
0:09:29
 0:09:34
0:09:34
 0:42:44
0:42:44
 0:03:20
0:03:20
 0:07:57
0:07:57
 0:05:36
0:05:36
 0:04:12
0:04:12
 0:03:56
0:03:56
 0:08:31
0:08:31
 0:05:51
0:05:51
 0:06:48
0:06:48
 0:06:48
0:06:48
 0:07:35
0:07:35
 0:01:57
0:01:57
 0:07:54
0:07:54
 0:32:08
0:32:08
 0:08:12
0:08:12
 0:09:34
0:09:34