filmov
tv
How to Write the Equation for CuF2 + H2O

Показать описание
In this video we will describe the equation CuF2 + H2O and write what happens when CuF2 is dissolved in water. The answer is that it is slightly soluble in water. Although it is an ionic compound, it does not dissociate much into its ions in water, however a small amount does dissolve.
When this small amount of CuF2 is dissolved in H2O (water) it will dissociate (dissolve) into Cu 2+ and F- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water.
The equation for CuF2,Copper (II) fluoride, and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Cu 2+ and F- back to CuF2 (just let the H2O evaporate). At the same time, the CuF2 is a very different substance than Cu 2+ and F-.
If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations:
When this small amount of CuF2 is dissolved in H2O (water) it will dissociate (dissolve) into Cu 2+ and F- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water.
The equation for CuF2,Copper (II) fluoride, and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Cu 2+ and F- back to CuF2 (just let the H2O evaporate). At the same time, the CuF2 is a very different substance than Cu 2+ and F-.
If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations:
 0:05:43
0:05:43
 0:02:40
0:02:40
 0:21:56
0:21:56
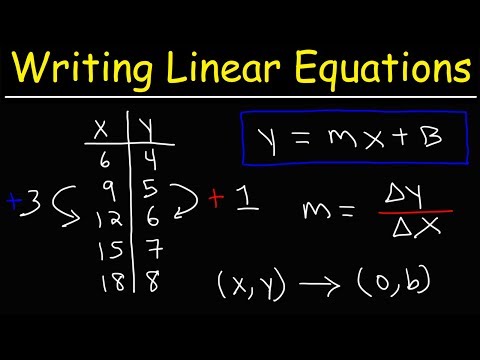 0:14:51
0:14:51
 0:03:38
0:03:38
 0:11:41
0:11:41
 0:12:52
0:12:52
 0:08:49
0:08:49
 0:05:18
0:05:18
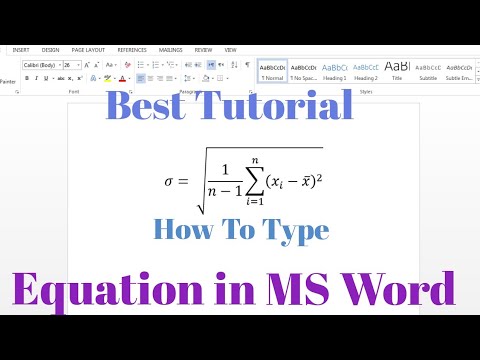 0:04:07
0:04:07
 0:02:09
0:02:09
 0:10:43
0:10:43
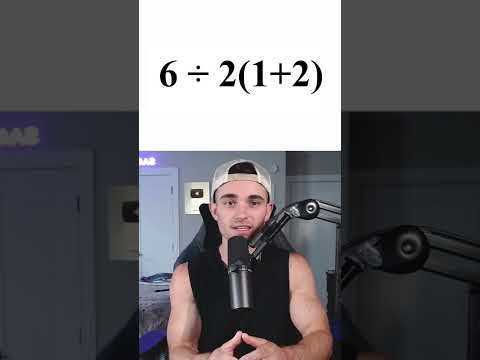 0:00:28
0:00:28
 0:04:12
0:04:12
 0:10:05
0:10:05
 0:08:32
0:08:32
 0:05:26
0:05:26
 0:00:21
0:00:21
 0:02:19
0:02:19
 0:00:16
0:00:16
 0:00:52
0:00:52
 0:00:23
0:00:23
 0:00:13
0:00:13
 0:32:05
0:32:05