filmov
tv
R.3.4.2 Nucleophilic Substitution Reactions [SL IB CHEMISTRY]

Показать описание
Enjoy.
You do not need to know any mechanisms for SL
You do not need to know any mechanisms for SL
R3.4.2 Nucleophilic substitution reactions
10.2 Nucleophilic Substitution [SL IB Chemistry]
15 Nucleophilic Substitution
Nucleophilic Substitution I (IB Chemistry R3.4)
20.2.3 Describe the rate of nucleophilic substitution in halogenoalkanes
10.2 Reactions of the halogenoalkanes (SL)
Nucleophilic Substitution | A Level & IB Chemistry
R3.4.10 Describe and explain rates of nucleophilic substitution [HL IB Chemistry]
10.5.1/10.5.2 Substitution nucleophilic reactions
Mechanisms and Reactivity of Halogenoalkanes
Nucleophilic Substitution 2A
20.2.2 Describe and explain how the rate of SN reactions depends on the identity of the halogen.
7 Reactions of Halogen Compounds Nucleophilic Substitution
IB Chemistry-SN2 Reaction:Nucleophilic substitution
Nucleophilic Substitution Reactions (SN2) MADE EASY - GAMSAT Science
Halogenoalkanes Nucleophilic Substitution A level Chemistry
Electrophilic Substitution (IB Chemistry R3.4)
Secondary Halogenoalkane Part 4: SN1 & SN2
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement
Electron-pair Sharing Reactions [IB Chemistry SL/HL]
SN1 SN2 Mechanisms Haloalkanes Organic Chemistry Best Concepts +Tricks TAMIL Class 11&12
An Animated Explanation of Electrophilic Addition
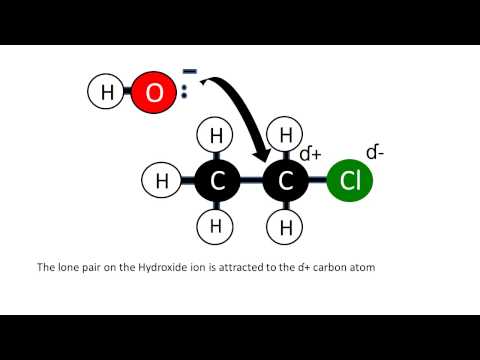
Nucleophilic Substitution with Hydroxide Ions
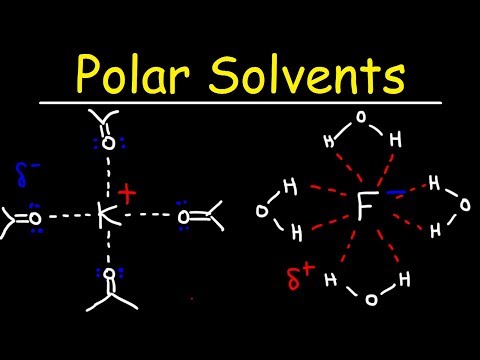
Polar Protic Solvents and Polar Aprotic Solvents For SN1 & SN2 Reactions
Комментарии
 0:02:59
0:02:59
 0:04:25
0:04:25
 0:09:07
0:09:07
 0:21:16
0:21:16
 0:01:46
0:01:46
 0:02:49
0:02:49
 0:07:03
0:07:03
 0:02:32
0:02:32
 0:05:30
0:05:30
 0:19:27
0:19:27
 0:04:17
0:04:17
 0:01:25
0:01:25
 0:00:58
0:00:58
 0:05:16
0:05:16
 0:05:51
0:05:51
 0:14:52
0:14:52
 0:18:12
0:18:12
 0:16:06
0:16:06
 0:34:45
0:34:45
 0:08:48
0:08:48
 0:34:46
0:34:46
 0:05:34
0:05:34
 0:01:38
0:01:38
 0:15:18
0:15:18