filmov
tv
Writing an Equation for an Isotope Produced by Bombardment

Показать описание
Follow us:
Q1. Write the balanced nuclear equation for the bombardment of nickel-58 by a proton, (_1^1)H, which produces a radioactive isotope and an alpha particle.
Q2. The first radioactive isotope was produced in 1934 by the bombardment of aluminum-27 by an alpha particle to produce a radioactive isotope and one neutron. Write the balanced nuclear equation for this bombardment.
Step 1 Write the incomplete nuclear equation.
Step 2 Determine the missing mass number. In the equation, the sum of the mass numbers of the proton, 1, and the nickel, 58, must equal the sum of the mass numbers of the new nucleus and the alpha particle.
1 + 58 = ? + 4
59 – 4 = ?
59 – 4 = 55 (mass number of new nucleus)
Step 3 Determine the missing atomic number. The sum of the atomic numbers of the proton, 1, and nickel, 28, must equal the sum of the atomic numbers of the new nucleus and the alpha particle.
1 + 28 = ? + 2
29 – 2 = ?
29 – 2 = 27 (atomic number of new nucleus)
Step 4 Determine the symbol of the new nucleus. On the periodic table, the element that has atomic number 27 is cobalt, Co. The nucleus of this isotope of Co is written as
Step 5 Complete the nuclear equation.
Комментарии
 0:02:40
0:02:40
 0:03:25
0:03:25
 0:03:06
0:03:06
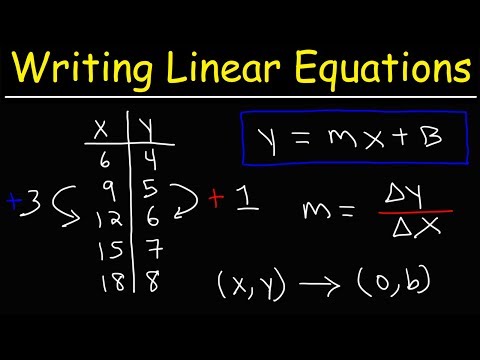 0:14:51
0:14:51
 0:10:05
0:10:05
 0:02:09
0:02:09
 0:06:54
0:06:54
 0:03:38
0:03:38
 0:01:01
0:01:01
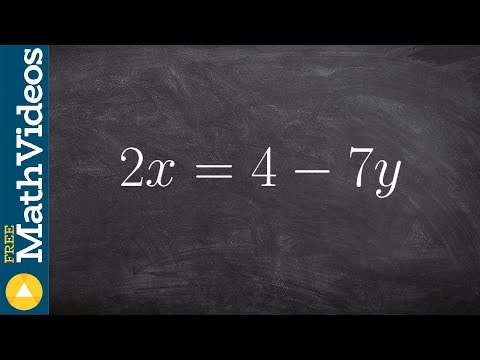 0:02:19
0:02:19
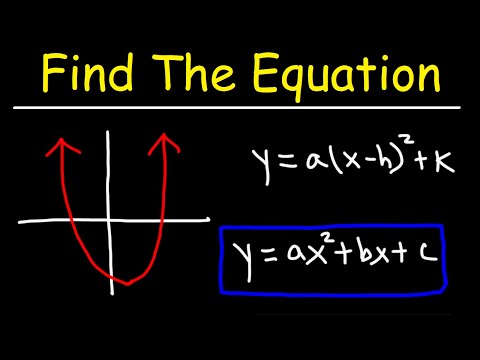 0:09:35
0:09:35
 0:01:03
0:01:03
 0:08:32
0:08:32
 0:08:40
0:08:40
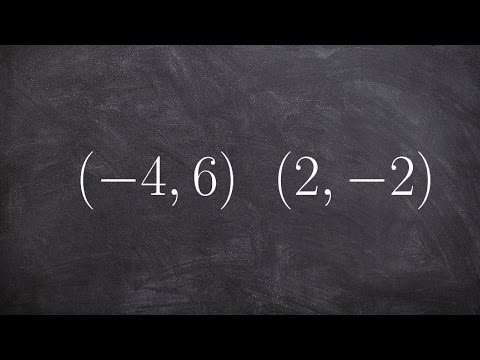 0:03:43
0:03:43
 0:02:47
0:02:47
 0:06:15
0:06:15
 0:07:53
0:07:53
 0:04:37
0:04:37
 0:24:28
0:24:28
 0:04:07
0:04:07
 0:03:20
0:03:20
 0:02:03
0:02:03
 0:02:18
0:02:18