filmov
tv
14.1 sigma and pi bonds (HL) old version

Показать описание
Updated video can be found here:
This video covers the formation of sigma and pi bonds.
Please note that I made in mistake in calling ethane, ethene. Ethane has a carbon to carbon single bond, ethene has a carbon to carbon double bond.
Help support this channel:
This video covers the formation of sigma and pi bonds.
Please note that I made in mistake in calling ethane, ethene. Ethane has a carbon to carbon single bond, ethene has a carbon to carbon double bond.
Help support this channel:
Topic 14. 1 - Sigma and pi bonding (5 and 6 electron domains)
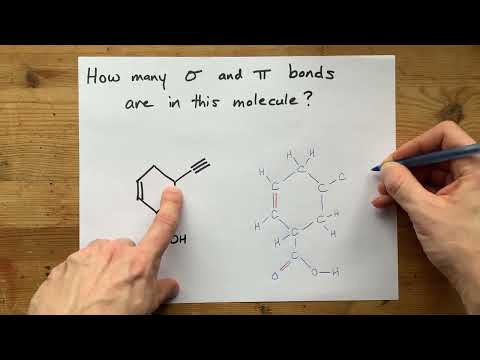
How many Sigma and Pi bonds are in this molecule?
Super Trick To Calculate sigma and Pi Bond | Organic Chemistry Class 11 | JEE / NEET | ATP STAR KOTA
Sigma and Pi Bond Symmetry
Sigma and Pi bonds in Alkenes - Exam Skills and Tips!
U8CE Hybridzation and Sigma Pi Bonds
Sigma and Pi bonds - Organic Chemistry Made Simple
TRICK to find sigma and pi bond #shorts #youtubeshorts #neet #chemistry
Organometallic Chemistry for IIT JAM 2025 | Must-Solve Questions!
The number of sigma (σ) and pi (π) bonds present in 1,3,5, 7-octatetraene respectively are : (a) ......
Identifying Sigma and Pi bonds
AS Chemistry Bonding — Sigma & Pi Bonds
Sigma Bonds Overlappings #dur_muhammad
Hybridisation of C Atom | Sigma & Pi Bond Calculation
How many sigma bonds are in Methane ? #chemistry#short
Covalent Bond explained #shorts #chemistry #jeemains #neet
Sigma and Pi Bonds in Organic Molecules
8.6 | How many σ and π bonds are present in the molecule HCN?
Sigma and Pi bonding - Chemical Bonding And Molecular Structure (Part 14)
9.4 Sigma and Pi Bonds
Chemical Bonding for A'Levels : Part 1 : Sigma Bonds
sigma donor-pi donor ligands
Sigma and Pi Bond | GOC -03 | Best Trick | Class-11th | Organic Chemistry | JEE | NEET | AIIMS
The number of sigma and Pi bonds in a molecule of cyanogen are: a. 4,3 b. 3,4 C. 5,2
Комментарии
 0:15:40
0:15:40
 0:04:22
0:04:22
 0:05:22
0:05:22
 0:25:55
0:25:55
 0:04:32
0:04:32
 0:09:50
0:09:50
 0:07:30
0:07:30
 0:00:56
0:00:56
 2:00:21
2:00:21
 0:02:00
0:02:00
 0:05:50
0:05:50
 0:10:58
0:10:58
 0:00:20
0:00:20
 0:12:43
0:12:43
 0:00:15
0:00:15
 0:00:26
0:00:26
 0:15:25
0:15:25
 0:07:07
0:07:07
 0:11:13
0:11:13
 0:07:08
0:07:08
 0:19:52
0:19:52
 0:00:16
0:00:16
 0:26:48
0:26:48
 0:00:50
0:00:50