filmov
tv
Blocks in Periodic Table | Chemistry Coaching By Laiba Aasim

Показать описание
Understanding the Blocks in the Periodic Table
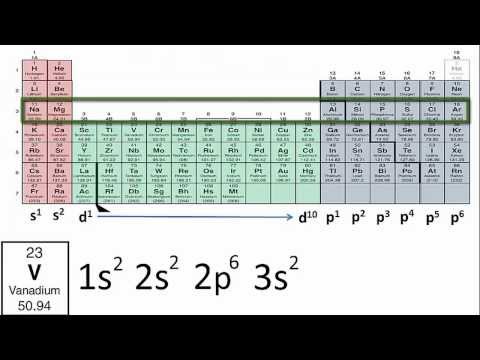
In this video, we explore the concept of blocks in the periodic table. The periodic table is divided into four main blocks — s-block, p-block, d-block, and f-block — based on the subshell in which the outermost electrons of the elements reside.
- **s-block** elements, which include Groups 1 and 2 (alkali metals and alkaline earth metals), have their outermost electrons in the s-orbital.
- **p-block** elements (Groups 13 to 18) have their outermost electrons in the p-orbital, and this block includes diverse elements like metals, metalloids, and nonmetals.
- **d-block** elements, also known as the transition metals, occupy Groups 3 to 12 and have their outermost electrons in the d-orbital.
- **f-block** elements, known as the inner transition metals, include the lanthanides and actinides, with their outermost electrons in the f-orbital.
#PeriodicTable
#Chemistry
#ElectronConfiguration
#StudyChemistry
In this video, we explore the concept of blocks in the periodic table. The periodic table is divided into four main blocks — s-block, p-block, d-block, and f-block — based on the subshell in which the outermost electrons of the elements reside.
- **s-block** elements, which include Groups 1 and 2 (alkali metals and alkaline earth metals), have their outermost electrons in the s-orbital.
- **p-block** elements (Groups 13 to 18) have their outermost electrons in the p-orbital, and this block includes diverse elements like metals, metalloids, and nonmetals.
- **d-block** elements, also known as the transition metals, occupy Groups 3 to 12 and have their outermost electrons in the d-orbital.
- **f-block** elements, known as the inner transition metals, include the lanthanides and actinides, with their outermost electrons in the f-orbital.
#PeriodicTable
#Chemistry
#ElectronConfiguration
#StudyChemistry
 0:01:55
0:01:55
 0:11:44
0:11:44
 0:02:05
0:02:05
 0:09:18
0:09:18
 0:07:23
0:07:23
 0:03:12
0:03:12
 0:11:14
0:11:14
 0:07:44
0:07:44
 3:38:38
3:38:38
 0:04:52
0:04:52
 0:08:27
0:08:27
 0:11:10
0:11:10
 0:14:28
0:14:28
 0:24:09
0:24:09
 0:04:01
0:04:01
 0:00:11
0:00:11
 0:14:39
0:14:39
 0:04:05
0:04:05
 0:00:37
0:00:37
 0:00:54
0:00:54
 0:00:05
0:00:05
 0:00:14
0:00:14
 0:00:23
0:00:23
 0:00:57
0:00:57