filmov
tv
18.2 Calculating pH of weak acids and bases (HL)
Показать описание
Applications and skills:
Calculations involving pH, pOH, Ka and Kb.
Calculations involving pH, pOH, Ka and Kb.
pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
pH of a Weak Acid Example 2: pH of a 0.50 M Hypochlorous Acid Solution
Chem 116 Ch 18 finding pH of weak acids and bases (new)
Finding pH of Weak Acids - KA constant, Buffers, and ICE Tables
pH of a Weak Base
Calculating the pH of Weak Acids and Weak Bases
Calculating the pH of a weak diprotic acid solution
CHEMISTRY 201: Calculating pH of a weak polyprotic acid
Finding pH for a weak base using kb ex 1 2
How to Calculate the pH of a Weak Acid Solution
Weak Acid / Strong Base Titration - All pH Calculations
Calculating the pH of a Weak Acid in Equilibrium (ICE and quadratic)
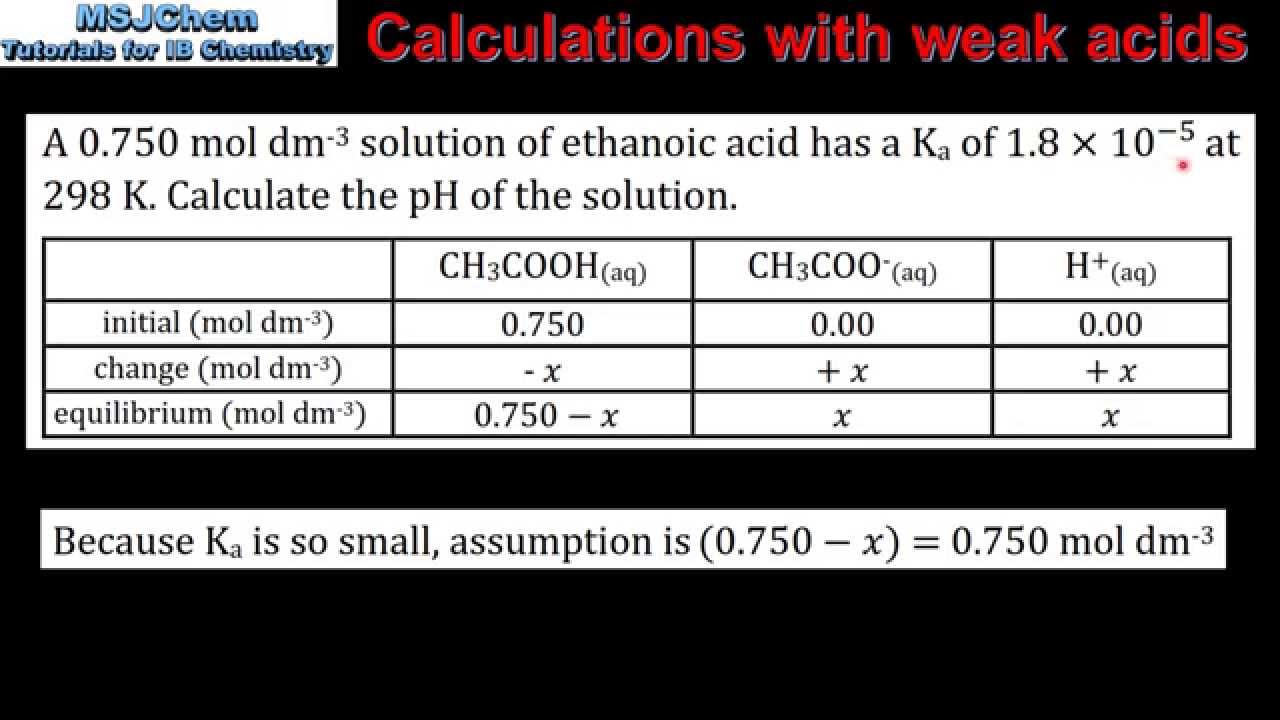
A 0 185 M solution of a weak acid HA has a pH of 2 95 Calculate the acid ionization constant Ka for
pH of a weak acid and weak base
pH of Weak Acids
pH of a weak acid
pH Calculation for Weak Acids and Bases
Salts of Weak Bases and Strong Acids - pH of a Solution Computation
Calculate Kb From pH of a Weak Base Solution 005
Calculation of pH and the extent of ionization of a weak electrolyte
How to Determine the Amount of a Weak Acid Needed to Produce a Certain pH
pH calculations for very weak acids
Weak Acid pH Calculations and Problems SIMPLIFIED
pH of a weak base and equimolar strong acid
Комментарии
 0:29:31
0:29:31
 0:01:43
0:01:43
 0:19:03
0:19:03
 0:07:59
0:07:59
 0:03:00
0:03:00
 0:09:47
0:09:47
 0:07:34
0:07:34
 0:06:02
0:06:02
 0:04:05
0:04:05
 0:08:32
0:08:32
 0:18:52
0:18:52
 0:15:30
0:15:30
 0:04:31
0:04:31
 0:06:25
0:06:25
 0:09:48
0:09:48
 0:04:42
0:04:42
 0:08:41
0:08:41
 0:20:30
0:20:30
 0:08:27
0:08:27
 0:02:38
0:02:38
 0:13:00
0:13:00
 0:16:29
0:16:29
 0:22:31
0:22:31
 0:05:50
0:05:50