filmov
tv
11.3 Structures of Solids | General Chemistry
Показать описание
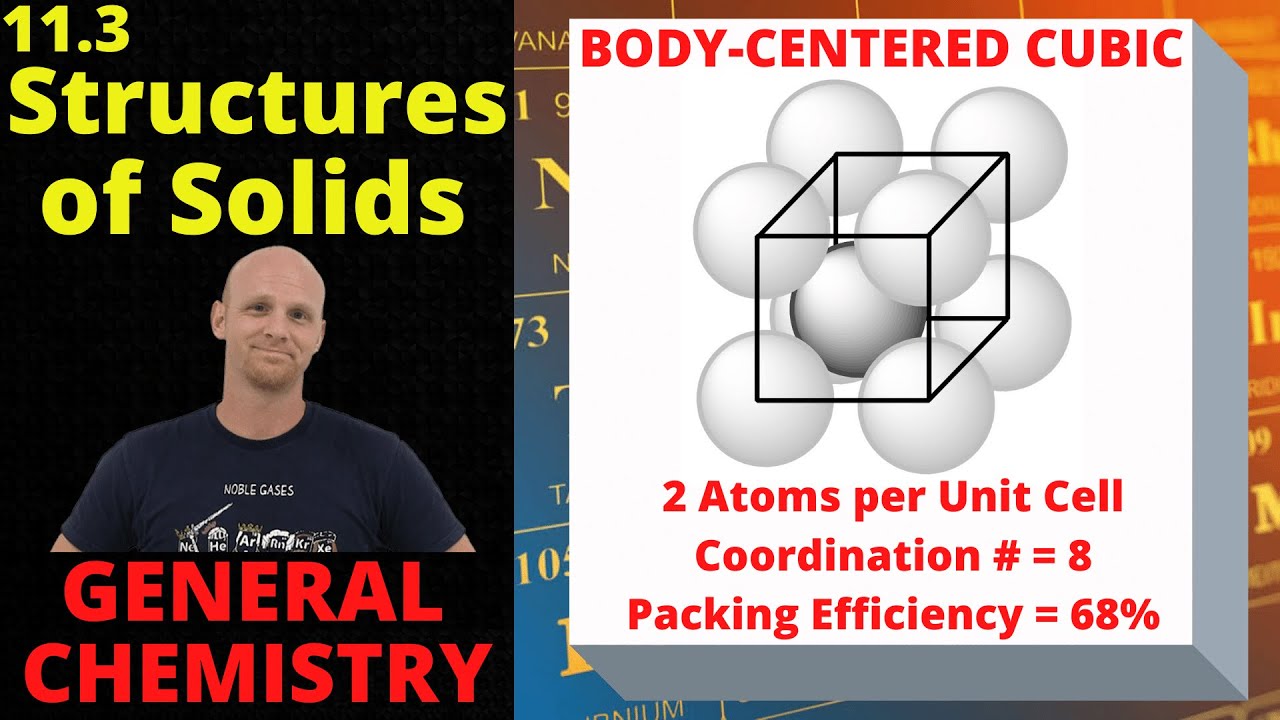
Chad provides a lesson on the classification and structures of solids. Amorphous solids are briefly defined before a discussion of crystalline solids with cubic unit cells including Simple Cubic (aka Primitive Cubic), Body-Centered Cubic (BCC), and Face-Centered Cubic (FCC). In these three types of cubic unit cells, the positions occupied by the atoms, the number of atoms per unit cell, the coordination number, and the packing efficiency are all compared and contrasted. A brief discussion of close-packing of spheres is included with a comparison of Hexagonal Close-Packing and Cubic Close-Packing structures follows (HCP vs CCP).
The lesson is concluded with a discussion of the bonding in four different types of solids and how this affects their melting points. Molecular solids are held together by relatively weak intermolecular forces and therefore tend to have relatively low melting points. In contrast, Network Covalent solids and Ionic solids are held together by covalent and ionic bonds respectively and typically have significantly higher melting points. Finally, Metallic solids are held together by metallic bonding. A wide range of strengths are possible in metallic bonding and is related to the number of valence electrons and the number of electrons available for bonding, and therefore the melting points of metals can vary greatly.
00:00 Lesson Introduction
01:00 Simple Cubic, Body-Centered Cubic, and Face-Centered Cubic Unit Cells
16:12 Close-Packing of Spheres
20:58 Bonding in Solids
The lesson is concluded with a discussion of the bonding in four different types of solids and how this affects their melting points. Molecular solids are held together by relatively weak intermolecular forces and therefore tend to have relatively low melting points. In contrast, Network Covalent solids and Ionic solids are held together by covalent and ionic bonds respectively and typically have significantly higher melting points. Finally, Metallic solids are held together by metallic bonding. A wide range of strengths are possible in metallic bonding and is related to the number of valence electrons and the number of electrons available for bonding, and therefore the melting points of metals can vary greatly.
00:00 Lesson Introduction
01:00 Simple Cubic, Body-Centered Cubic, and Face-Centered Cubic Unit Cells
16:12 Close-Packing of Spheres
20:58 Bonding in Solids
Комментарии
 0:20:42
0:20:42
 0:20:38
0:20:38
 0:04:35
0:04:35
 0:25:43
0:25:43
 0:18:30
0:18:30
 0:12:55
0:12:55
 0:20:19
0:20:19
 0:12:46
0:12:46
 0:01:52
0:01:52
 0:08:33
0:08:33
 0:14:28
0:14:28
 1:15:12
1:15:12
 0:25:08
0:25:08
 0:12:27
0:12:27
 0:20:13
0:20:13
 0:11:40
0:11:40
 0:12:17
0:12:17
 0:12:53
0:12:53
 0:07:02
0:07:02
 0:02:30
0:02:30
 0:17:04
0:17:04
 0:38:40
0:38:40
 0:40:57
0:40:57
 0:23:11
0:23:11