filmov
tv
Calculating pH From [H₃O⁺] in Aqueous Solution 001

Показать описание
Calculate the pH of a solution if the [H3O+] = 3.0 x 10-4 M.
⚗️ Calculating pH from [H₃O⁺] or [OH⁻]
Calculating pH From [H₃O⁺] in Aqueous Solution 001
Worked examples: Calculating [H₃O⁺] and pH | Acids and bases | AP Chemistry | Khan Academy
⚗️ Calculating [H₃O⁺] from pH
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
Calculating pH from [H3O+]
Calculate pH from H+ concentration 008
Calculating pH from [H3O+] or [OH-]
Calculating pH from [OH-]
Calculating pH from [H3O]
h3o and ph calculations
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 06
Calculate [OH⁻] of H₃O⁺ Solution - acidic, basic, neutral 007
LM 11 Calculating pH from [H+]
Calculate pH from [H3O+] and vice versa
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 09
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 08
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 12
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 07
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 10
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 11
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 04
CW: pH, pOH, [OH⁻], & [H₃O⁺] Calculations 01
pH Calculations: From pH to [H3O+]
Комментарии
 0:04:35
0:04:35
 0:02:06
0:02:06
 0:07:05
0:07:05
![⚗️ Calculating [H₃O⁺]](https://i.ytimg.com/vi/GqXArRFQ1cU/hqdefault.jpg) 0:03:01
0:03:01
 0:13:50
0:13:50
 0:03:11
0:03:11
 0:01:54
0:01:54
 0:10:40
0:10:40
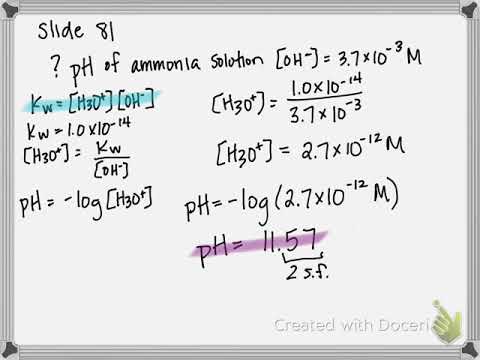 0:05:42
0:05:42
 0:00:55
0:00:55
 0:06:31
0:06:31
 0:01:34
0:01:34
![Calculate [OH⁻] of](https://i.ytimg.com/vi/fdqUiDvRamE/hqdefault.jpg) 0:02:33
0:02:33
 0:02:00
0:02:00
 0:02:13
0:02:13
 0:01:39
0:01:39
 0:01:35
0:01:35
 0:01:35
0:01:35
 0:01:37
0:01:37
 0:01:34
0:01:34
 0:01:37
0:01:37
 0:01:34
0:01:34
 0:01:40
0:01:40
 0:09:01
0:09:01