filmov
tv
Given figure shows how the temperature of water and ice mixed with each other at different temper...

Показать описание
Given figure shows how the temperature of water and ice
mixed with each other at different temperatures, changes
with the amount of heat (Q) evolved by the water or absorbed
by the ice. The scale corresponding to heat (Q) is arbitrary.
Which of the following is/are conclusions drawn from the
graph?
(1) The mass of water is double that of ice.
(2) The equilibrium temperature will be 0^∘C, with threefourth of the ice melted.
(3) The equilibrium temperature will be 0^∘C with one-fourth of the water frozen.
(4) The equilibrium temperature will be 10^∘C.
📌 PHYSICS WALLAH OTHER CHANNELS :
📌 PHYSICS WALLAH SOCIAL MEDIA PROFILES :
mixed with each other at different temperatures, changes
with the amount of heat (Q) evolved by the water or absorbed
by the ice. The scale corresponding to heat (Q) is arbitrary.
Which of the following is/are conclusions drawn from the
graph?
(1) The mass of water is double that of ice.
(2) The equilibrium temperature will be 0^∘C, with threefourth of the ice melted.
(3) The equilibrium temperature will be 0^∘C with one-fourth of the water frozen.
(4) The equilibrium temperature will be 10^∘C.
📌 PHYSICS WALLAH OTHER CHANNELS :
📌 PHYSICS WALLAH SOCIAL MEDIA PROFILES :
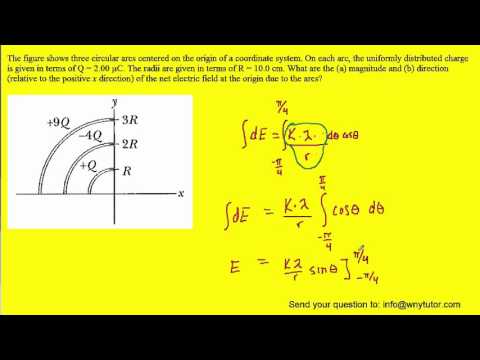
The figure shows three circular arcs centered at the origin of a coordinate system
The given figure shows an estimated force-time graph for a baseball struck by . . .
Math 1A HW 2.7.41 The figure shows the graphs of f, f ', and f ''. Identify each curv...
The figure given alongside shows the path of a diver, when she takes a jump from the diving board.
Figure shows the strain-stress curve for a given material. What are...
The figure shows four penguins that are being playfully pulled along
Consider the following figure find the equivalent capacitance
The given figure shows an example of;\n....
HCV: Figure shows a man of mass 60 kg standing on a light weighing machine kept in a box of mass 30
JEE ADVANCED 2022 paper 1 question 10 the figure shows a circuit having eight resistances of 1Ω each...
The figure given alongside shows the path of a diver, when she takes a jump from the diving board.
the magnitude of vectors OA,OB and OC in the given figure are equal the direction of OA+OB-OC with X
A particle shows distance-time curve as given in this figure. The maximum instantaneous velocity of
HCV: Figure shows the graph of velocity versus time for a particle going along the X-axis. Find
The figure shows position-time graph of two Riders C and D. Based on the...| Krishna Ke Doubts
Figure shows a long straight wire of a circular crosssection (radiu...
The figure shows a plot of terminal voltage \'V\' verus the current \'I\' of a g...
Figure shows a set of equipotential surfaces. The magnitude and direction of electric
Figure shows electric field lines in which an electric dipole 𝐩 is placed as shown. Which of the......
5. Figure here shows electric field lines in which an electric dipole P is placed as shown
In the figure shown, blocks A and B move with velocities v1 and v2 along horizontal direction. Find
Name the angles in the given figure
PYQ JEE Advanced 2022 | Kailash Sir | The figure shows a circuit having eight resistances
if the diagram in figure shows the graph of the polynomial ax^2+bx+c | HOT #59 #term1|
Комментарии
 0:08:53
0:08:53
 0:05:34
0:05:34
 0:03:50
0:03:50
 0:13:22
0:13:22
 0:02:58
0:02:58
 0:06:52
0:06:52
 0:09:04
0:09:04
 0:02:24
0:02:24
 0:06:34
0:06:34
 0:04:29
0:04:29
 0:37:05
0:37:05
 0:05:05
0:05:05
 0:01:41
0:01:41
 0:03:17
0:03:17
 0:12:35
0:12:35
 0:04:13
0:04:13
 0:01:55
0:01:55
 0:04:56
0:04:56
 0:05:40
0:05:40
 0:02:02
0:02:02
 0:04:12
0:04:12
 0:01:57
0:01:57
 0:11:45
0:11:45
 0:02:51
0:02:51