filmov
tv
Chemical Bonding and Molecular Structure Chemistry Class 11 Chapter 4 - Covalent Bond
Показать описание
Chemical Bonding and Molecular Structure Chemistry Class 11 Chapter 4 - Covalent Bond
Topics covered in this 11th CBSE Chemistry video are as following:
Characteristics of Covalent bond:
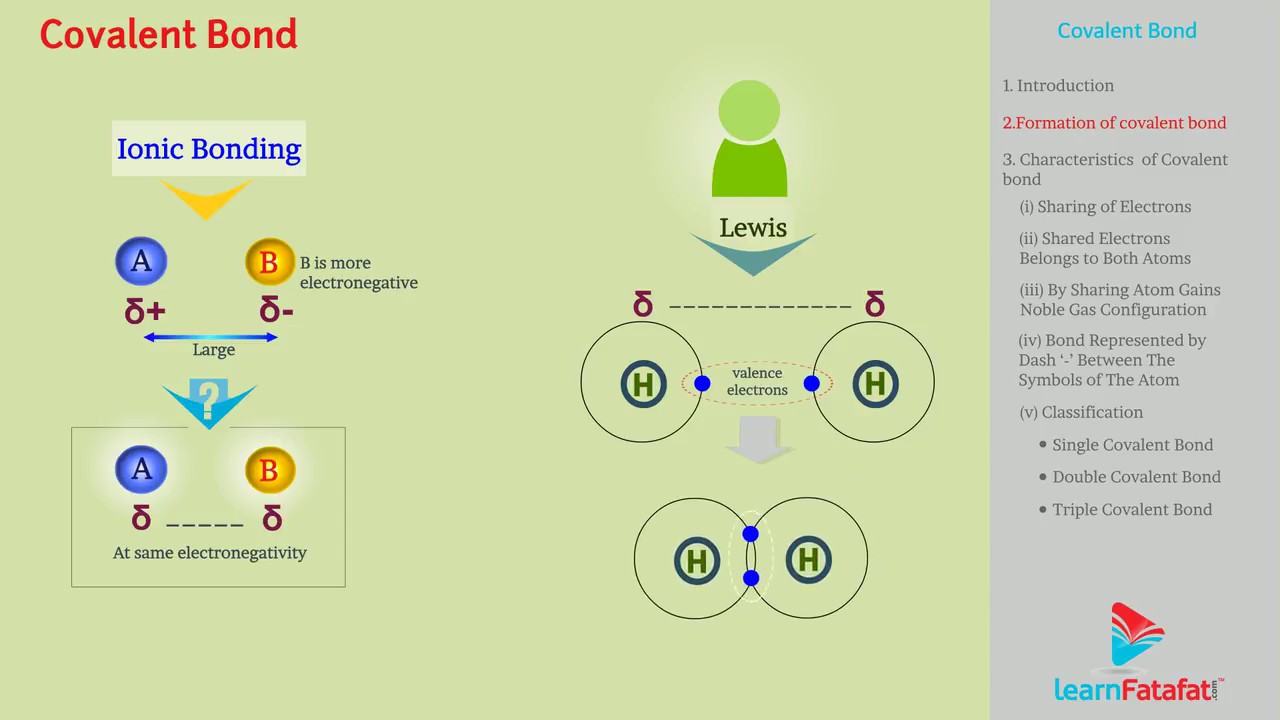
These are formed by sharing of equal number of unpaired electrons.
The shared electrons belongs to both atoms. So, each atom now have two electrons.
By sharing electrons each atom attains nearest noble gas configuration.
The bond formed by sharing of electron is represented by dash ‘-’ between the symbols of the atom. e.g. H-H.
Covalent bond can be classified as single covalent bond, double covalent bond or triple covalent bond.
Single Covalent Bond: The covalent bond formed by sharing of one electron each or one pair of electron between atoms, is called single covalent bond.
Double Covalent Bond: The covalent bond formed by sharing of two electrons each or two pairs of electrons between atoms is called Double covalent bond.
Triple Covalent Bond: The covalent bond formed by sharing of three electrons each or three pairs of electrons is called triple covalent bond.
Chemistry 11 Syllabus:
Chemistry I:
Chapter 1 Some Basic Concepts of Chemistry
Chapter 2 Structure of Atom
Chapter 3 Classification of Elements and Periodicity in Properties
Chapter 4 Chemical Bonding and Molecular Structure
Chapter 5 States of Matter
Chapter 6 Thermodynamics
Chapter 7 Equilibrium
Chemistry II:
Chapter 8 Redox Reactions
Chapter 9 Hydrogen
Chapter 10 The s-Block Elements
Chapter 11 The p-Block Elements
Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
Chapter 13 Hydrocarbons
Chapter 14 Environmental Chemistry
Topics covered in this 11th CBSE Chemistry video are as following:
Characteristics of Covalent bond:
These are formed by sharing of equal number of unpaired electrons.
The shared electrons belongs to both atoms. So, each atom now have two electrons.
By sharing electrons each atom attains nearest noble gas configuration.
The bond formed by sharing of electron is represented by dash ‘-’ between the symbols of the atom. e.g. H-H.
Covalent bond can be classified as single covalent bond, double covalent bond or triple covalent bond.
Single Covalent Bond: The covalent bond formed by sharing of one electron each or one pair of electron between atoms, is called single covalent bond.
Double Covalent Bond: The covalent bond formed by sharing of two electrons each or two pairs of electrons between atoms is called Double covalent bond.
Triple Covalent Bond: The covalent bond formed by sharing of three electrons each or three pairs of electrons is called triple covalent bond.
Chemistry 11 Syllabus:
Chemistry I:
Chapter 1 Some Basic Concepts of Chemistry
Chapter 2 Structure of Atom
Chapter 3 Classification of Elements and Periodicity in Properties
Chapter 4 Chemical Bonding and Molecular Structure
Chapter 5 States of Matter
Chapter 6 Thermodynamics
Chapter 7 Equilibrium
Chemistry II:
Chapter 8 Redox Reactions
Chapter 9 Hydrogen
Chapter 10 The s-Block Elements
Chapter 11 The p-Block Elements
Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
Chapter 13 Hydrocarbons
Chapter 14 Environmental Chemistry
 3:09:41
3:09:41
 1:21:51
1:21:51
 3:39:11
3:39:11
 5:23:20
5:23:20
 2:12:12
2:12:12
 1:31:18
1:31:18
 0:06:31
0:06:31
 0:24:44
0:24:44
 0:46:59
0:46:59
 0:52:52
0:52:52
 2:22:02
2:22:02
 0:09:46
0:09:46
 0:00:50
0:00:50
 3:15:11
3:15:11
 2:41:20
2:41:20
 0:27:34
0:27:34
 0:06:09
0:06:09
 0:56:40
0:56:40
 0:03:33
0:03:33
 0:00:20
0:00:20
 2:56:30
2:56:30
 0:11:38
0:11:38
 1:26:20
1:26:20
 1:03:55
1:03:55