filmov
tv
Introduction to Coulomb's Law + examples. Force between proton and electron in Hydrogen atom.

Показать описание
Questions or requests? Post your comments below, and I will respond within 24 hours.
In this introduction to Coulomb's law, we start by reviewing the direction of electric force for like and unlike charges. Next, we introduce Coulomb's law which describes the magnitude of the electric force as a function of separation distance and charge, in addition to describing the repulsion or attraction using a +/- sign, so Coulomb's Law governs the electrostatic force between two charges.
We finish by working two examples using Coulomb's law to calculate electrostatic force between point charges. In the first example, we find the electrical force between two +1 micro-Coulomb charges separated by a meter. In the second example, we calculate the force of attraction between the proton and electron in a hydrogen atom, assuming a classical atom and a diameter of 1 Angstrom. The electric force in the hydrogen atom provides centripetal force to curve the path of the electron into an orbit (in this simple classical model).
Coulomb's law
Coulomb's Law - Net Electric Force & Point Charges
Introduction to Coulomb's Law or the Electric Force
Introduction to Coulomb's Law
Intro to Coulombs Law
Electric Charge and Electric Fields
Introduction to Coulombs Law
Introductory physics II - Coulomb s Law introduction
Coulomb's Law
Physics 12.2.1b - Coulomb`s Law - Simple Examples
Coulomb's Law Explained: Basics, Force Direction & Examples
What Is Coulomb's Law? | Physics in Motion
1 Introduction Coulomb’sLaw
Electric Charge: Crash Course Physics #25
Coulombs law || 3D animated explanation || class 12th Physics || Electrostatics ||
Coulomb's Law Problems
Coulomb's Law (with example)
coulomb's law explained
Coulomb's Law Electrostatics grade 11 and 12
Coulombs Law Introduction
Electrostatics grade 11: Introduction
Understand Coulomb's Law in One Minute!! #ElectricalEngineering #Shorts
Coulomb's Law (1 of 7) An Explanation
Coulombs Law 1
Комментарии
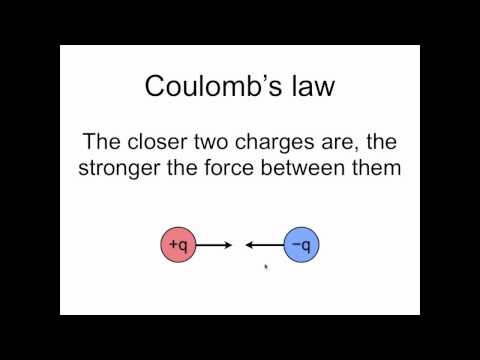 0:03:55
0:03:55
 0:35:53
0:35:53
 0:12:10
0:12:10
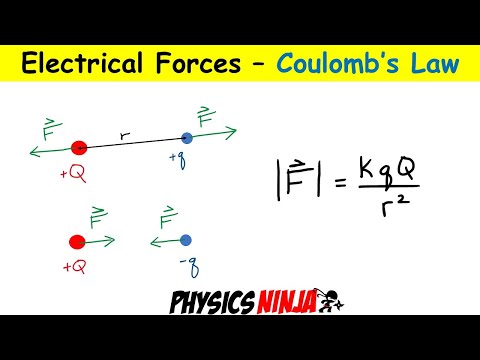 0:20:30
0:20:30
 0:06:39
0:06:39
 0:06:41
0:06:41
 0:10:02
0:10:02
 0:07:34
0:07:34
 0:16:13
0:16:13
 0:04:58
0:04:58
 0:13:02
0:13:02
 0:09:55
0:09:55
 0:08:08
0:08:08
 0:09:42
0:09:42
 0:02:35
0:02:35
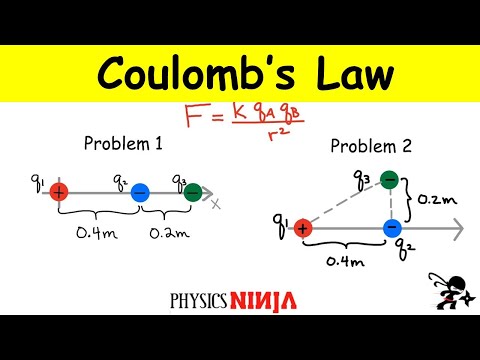 0:19:19
0:19:19
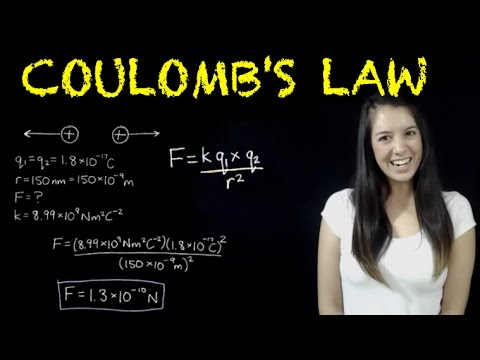 0:09:51
0:09:51
 0:12:22
0:12:22
 0:18:14
0:18:14
 0:07:50
0:07:50
 0:05:42
0:05:42
 0:01:00
0:01:00
 0:09:23
0:09:23
 0:25:49
0:25:49