filmov
tv
Why is There Absolute Zero Temperature? Why is There a Limit?

Показать описание
The highest temperature scientists obtained at the Large Hadron Collider is 5 trillion Kelvin.
The lowest temperature that people managed to obtain is 0.000000000038 (38 picoKelvin) or minus 273.14 degrees Celsius or minus 459.66 degrees Fahrenheit.
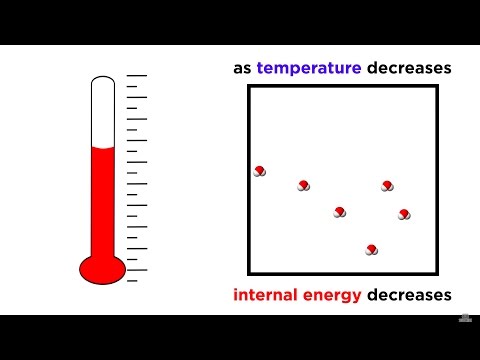
But is there anything else hotter or colder? What does "colder" or "hotter" mean? Why are some objects warmer than others? What is absolute zero, and why is it -459.67 Fahrenheit and -273.15 Celsius? Keep watching to learn this from the video!
Absolute zero.
#reyouniverse
The lowest temperature that people managed to obtain is 0.000000000038 (38 picoKelvin) or minus 273.14 degrees Celsius or minus 459.66 degrees Fahrenheit.
But is there anything else hotter or colder? What does "colder" or "hotter" mean? Why are some objects warmer than others? What is absolute zero, and why is it -459.67 Fahrenheit and -273.15 Celsius? Keep watching to learn this from the video!
Absolute zero.
#reyouniverse
Why is There Absolute Zero Temperature? Why is There a Limit?
Absolute Zero: Absolute Awesome
Neil deGrasse Tyson Explains Why You Can’t Reach Absolute Zero
Is It Possible To Get To Absolute Zero? | Neil deGrasse Tyson
Why Is Achieving EXACTLY Absolute Zero Impossible? | Neil deGrasse Tyson
What if Earth Dropped to Absolute Zero for 5 Seconds?
How Hot Can It Get?
The Completely Bizarre Physics At Near Absolute Zero
Noise Part 1. Noise in Microwave (High Freq) Circuit. Source of Noise: Internal & External Noise...
The Third Law of Thermodynamics: Absolute Zero
Do Electrons Stop Moving at Absolute Zero Temperature - What Happens at Absolute Zero Temperature
Temperature of these Black Holes is Absolute Zero 🤯
What Would Happen to Your Body at Absolute Zero
What If You Touched Absolute Zero?
Where does Absolute Zero come from?
What’s The Hottest Hot and Coldest Cold?
Do electrons move at Absolute Zero?
Quantum Cooling to (Near) Absolute Zero
What are Superfluids and Why Are They Important?
Does Absolute Zero Temperature Occur In The Universe? | Abhijit Chavda
I Calculated Absolute Zero With Vodka
What is Absolute Zero? Searching for the Coldest Possible Temperature
Experiment to determine absolute zero
Absolute Zero!🤧 #shorts
Комментарии
 0:15:48
0:15:48
 0:03:16
0:03:16
 0:17:11
0:17:11
 0:01:00
0:01:00
 0:01:00
0:01:00
 0:07:10
0:07:10
 0:10:03
0:10:03
 0:17:10
0:17:10
 0:15:26
0:15:26
 0:03:41
0:03:41
 0:00:57
0:00:57
 0:00:33
0:00:33
 0:09:35
0:09:35
 0:05:26
0:05:26
 0:03:58
0:03:58
 0:05:47
0:05:47
 0:08:26
0:08:26
 0:09:57
0:09:57
 0:07:11
0:07:11
 0:02:07
0:02:07
 0:16:51
0:16:51
 0:06:56
0:06:56
 0:04:25
0:04:25
 0:00:46
0:00:46