filmov
tv
Nucleophilic substitution reaction|| SN1 and SN2 reactions#youtubeshorts #chemistry #foryou

Показать описание
#advancedchemistry #youtubeshorts #chemistry #electrophilicsubstitution #organicreaction #chemistrynotes #yt #nucleophilicsubstitutionreaction #nucleophilic #nucleophile #electrophile #science #science #viralvideo #viralshorts #unfreezemyacount #views_viral_video_subscribers_grow #viewsplz #views #carbocation #bonding #fscchemistry #fsc #fscpart2 #mdcat2021syllabus #mdcat2024 #mdcat #neet #explore #fypシ゚viral #chemistryeducation #chemistryclass12 #organicchemistry12 #organicchemistry #organicreaction #organic #followme #explorepage #hyperconjugation #resonanceeffect #learning #lesson #mechanism #inductiveeffecttrick #tricks #ytshort #ytshortsindia #sn1reaction #sn2reaction
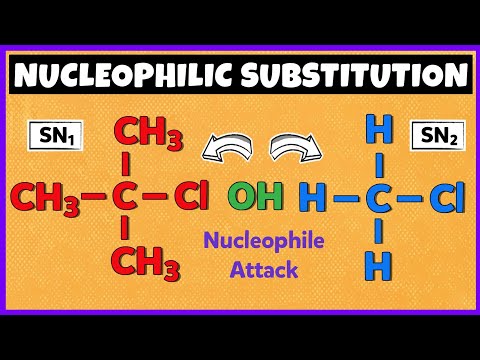
SN1 and SN2 are two types of nucleophilic substitution reactions, a fundamental concept in organic chemistry. The main difference between them lies in the mechanism, rate-determining step, and stereochemistry:
SN1 Reaction:
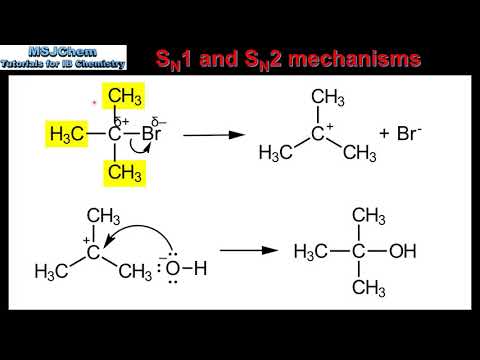
1. Unimolecular, rate-determining step: Formation of a carbocation intermediate.
2. Two-step process:
- Step 1: Leaving group departure forms a carbocation.
- Step 2: Nucleophile attacks the carbocation.
3. Stereochemistry: Racemic mixture (50:50) of enantiomers, due to the planar carbocation intermediate.
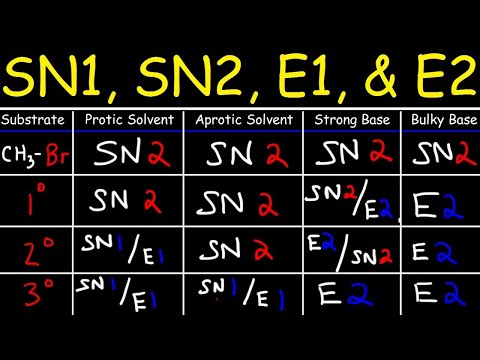
4. Rate depends on substrate and leaving group.
5. Typically occurs with tertiary substrates (3°) and good leaving groups.
SN2 Reaction:
1. Bimolecular, concerted mechanism: Nucleophile attacks and leaving group departs simultaneously.
2. One-step process.
3. Stereochemistry: Inversion of configuration (Walden inversion) due to backside attack by the nucleophile.
4. Rate depends on substrate, nucleophile, and leaving group.
5. Typically occurs with primary (1°) and secondary (2°) substrates, and good nucleophiles.
Key differences:
- SN1: Two-step, carbocation intermediate, racemic mixture
- SN2: One-step, concerted, inversion of configuration
Understanding SN1 and SN2 reactions helps predict reaction outcomes, choose appropriate reaction conditions, and design synthesis routes.
Do you have any specific questions about SN1 and SN2 reactions or would you like further clarification?
SN1 and SN2 are two types of nucleophilic substitution reactions, a fundamental concept in organic chemistry. The main difference between them lies in the mechanism, rate-determining step, and stereochemistry:
SN1 Reaction:
1. Unimolecular, rate-determining step: Formation of a carbocation intermediate.
2. Two-step process:
- Step 1: Leaving group departure forms a carbocation.
- Step 2: Nucleophile attacks the carbocation.
3. Stereochemistry: Racemic mixture (50:50) of enantiomers, due to the planar carbocation intermediate.
4. Rate depends on substrate and leaving group.
5. Typically occurs with tertiary substrates (3°) and good leaving groups.
SN2 Reaction:
1. Bimolecular, concerted mechanism: Nucleophile attacks and leaving group departs simultaneously.
2. One-step process.
3. Stereochemistry: Inversion of configuration (Walden inversion) due to backside attack by the nucleophile.
4. Rate depends on substrate, nucleophile, and leaving group.
5. Typically occurs with primary (1°) and secondary (2°) substrates, and good nucleophiles.
Key differences:
- SN1: Two-step, carbocation intermediate, racemic mixture
- SN2: One-step, concerted, inversion of configuration
Understanding SN1 and SN2 reactions helps predict reaction outcomes, choose appropriate reaction conditions, and design synthesis routes.
Do you have any specific questions about SN1 and SN2 reactions or would you like further clarification?
 0:08:03
0:08:03
 0:12:19
0:12:19
 0:03:30
0:03:30
 0:05:16
0:05:16
 1:13:40
1:13:40
 0:26:25
0:26:25
 0:24:19
0:24:19
 0:08:45
0:08:45
 0:20:08
0:20:08
 0:02:25
0:02:25
 0:06:05
0:06:05
 0:13:31
0:13:31
 0:12:07
0:12:07
 0:38:50
0:38:50
 0:10:35
0:10:35
 1:03:59
1:03:59
 0:00:05
0:00:05
 0:00:32
0:00:32
 0:03:58
0:03:58
 0:06:17
0:06:17
 0:28:42
0:28:42
 0:00:21
0:00:21
 0:12:11
0:12:11
 0:06:43
0:06:43