filmov
tv
Redox: Oxidation & Reduction | A-level Chemistry | OCR, AQA, Edexcel

Показать описание
Redox: Oxidation & Reduction in a Snap!
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Oxidation and Reduction
2. Electron Transfer
3. Redox Reactions
4. Metals with Acids
Reminder: Oxidation and Reduction
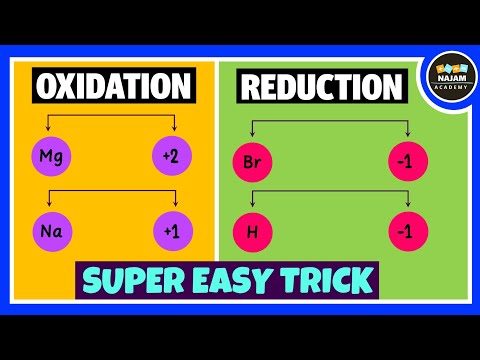
Can be considered in different contexts. Oxygen. Oxidation: Gain of Oxygen. Reduction: Loss of Oxygen. Electrons. Oxidation: Loss of electrons. Reduction: Gain of electrons.
Electron Transfer
This can be summarised as: OIL RIG. Oxidation is loss, Reduction is gain.
Redox Reactions
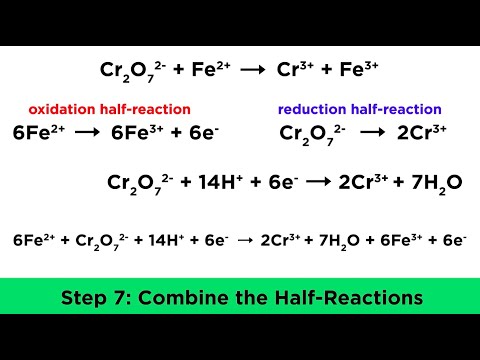
Reaction where oxidation and reduction occurs. Electrons are transferred. One species loses x electrons, Another species gains x electrons.
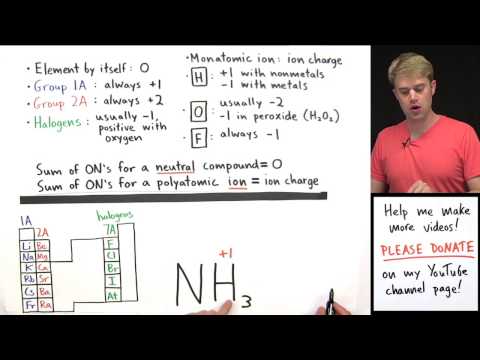
Oxidation Numbers
The charge the atom would have if it was composed of ions. Reflects the number of electrons that have been lost/gained. Oxidation: Increase in Oxidation Number. Reduction: Decrease in Oxidation Number.
Redox Reactions: Oxidation Numbers
Changes in oxidation number can reflect whether oxidation or reduction has occured. Oxidation: Increase in oxidation number. Reduction: Decrease in oxidation number.
Redox Reaction: Metals with Acids
Reactive metals react with acids. Metal is oxidised, Hydrogen is reduced to form Hydrogen Gas.
Oxidising and Reducing Agents
In our reactions we have Oxidising and Reducing Agents. They have different roles. Oxidising Agents. Oxidise other things, Are themselves reduced. Reducing Agents. Reduce other things, Are themselves oxidised.
Disproportionation Reactions
These are reactions where the same element is both oxidised and reduced. We can look at the changes in oxidation number to classify reactions. Example: The Decomposition of H2O2. The Oxygen is being both: Oxidised, Reduced
Summary
Oxidation numbers reflect the number of electrons that have been lost/gained
Each atom can be assigned an oxidation number
We use rules to work out oxidation numbers
a. Learn these!
In formulae:
a. In compounds:
i. The sum of the Oxidation Numbers = zero
b. In molecular ions:
i. The sum of the Oxidation Numbers = overall charge on the ion
Roman Numerals are used to represent oxidation states
Transition elements can take multiple oxidation numbers
Oxyanions are -ve ions, containing an oxygen and an element
a. End in -ate
b. Element can take multiple oxidation numbers
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Oxidation and Reduction
2. Electron Transfer
3. Redox Reactions
4. Metals with Acids
Reminder: Oxidation and Reduction
Can be considered in different contexts. Oxygen. Oxidation: Gain of Oxygen. Reduction: Loss of Oxygen. Electrons. Oxidation: Loss of electrons. Reduction: Gain of electrons.
Electron Transfer
This can be summarised as: OIL RIG. Oxidation is loss, Reduction is gain.
Redox Reactions
Reaction where oxidation and reduction occurs. Electrons are transferred. One species loses x electrons, Another species gains x electrons.
Oxidation Numbers
The charge the atom would have if it was composed of ions. Reflects the number of electrons that have been lost/gained. Oxidation: Increase in Oxidation Number. Reduction: Decrease in Oxidation Number.
Redox Reactions: Oxidation Numbers
Changes in oxidation number can reflect whether oxidation or reduction has occured. Oxidation: Increase in oxidation number. Reduction: Decrease in oxidation number.
Redox Reaction: Metals with Acids
Reactive metals react with acids. Metal is oxidised, Hydrogen is reduced to form Hydrogen Gas.
Oxidising and Reducing Agents
In our reactions we have Oxidising and Reducing Agents. They have different roles. Oxidising Agents. Oxidise other things, Are themselves reduced. Reducing Agents. Reduce other things, Are themselves oxidised.
Disproportionation Reactions
These are reactions where the same element is both oxidised and reduced. We can look at the changes in oxidation number to classify reactions. Example: The Decomposition of H2O2. The Oxygen is being both: Oxidised, Reduced
Summary
Oxidation numbers reflect the number of electrons that have been lost/gained
Each atom can be assigned an oxidation number
We use rules to work out oxidation numbers
a. Learn these!
In formulae:
a. In compounds:
i. The sum of the Oxidation Numbers = zero
b. In molecular ions:
i. The sum of the Oxidation Numbers = overall charge on the ion
Roman Numerals are used to represent oxidation states
Transition elements can take multiple oxidation numbers
Oxyanions are -ve ions, containing an oxygen and an element
a. End in -ate
b. Element can take multiple oxidation numbers
Комментарии
 0:16:05
0:16:05
 0:13:05
0:13:05
 0:03:52
0:03:52
 0:04:54
0:04:54
 0:11:13
0:11:13
 0:20:24
0:20:24
 0:07:31
0:07:31
 0:05:23
0:05:23
 0:21:25
0:21:25
 0:06:55
0:06:55
 0:03:56
0:03:56
 0:11:04
0:11:04
 0:14:56
0:14:56
 0:12:22
0:12:22
 0:07:17
0:07:17
 0:20:52
0:20:52
 0:26:28
0:26:28
 0:31:15
0:31:15
 0:16:00
0:16:00
 0:04:35
0:04:35
 0:07:04
0:07:04
 0:13:26
0:13:26
 0:13:55
0:13:55
 0:53:08
0:53:08