filmov
tv
Naming Molecular Compounds

Показать описание
Naming molecular compounds might sound tricky, but it's like learning a secret code to understand the language of chemistry. Molecular compounds are made up of nonmetals, and the way we name them helps us know exactly what elements are in them and how many.
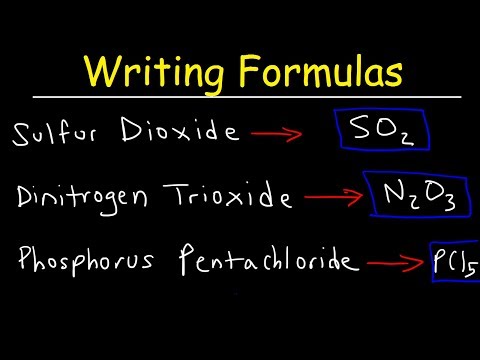
Here’s the deal: each compound has two parts. The first part of the name tells you the first element, and the second part tells you the second element with an “-ide” ending. For example, in carbon dioxide (CO₂), “carbon” is the first element, and “dioxide” tells you there are two oxygen atoms.
To make things clearer, we use prefixes to show how many atoms of each element are in the compound. These prefixes are: mono- (1), di- (2), tri- (3), tetra- (4), penta- (5), hexa- (6), and so on. But here’s a tip: if there’s only one atom of the first element, we usually skip the “mono-.” So, CO is carbon monoxide, not monocarbon monoxide.
Let’s break down another example: dinitrogen tetroxide (N₂O₄). “Di-” means two nitrogen atoms, and “tetra-” means four oxygen atoms.
Naming molecular compounds might seem like cracking a code, but once you know the rules, it’s a piece of cake. You’ll be able to decipher and create the names of compounds like a chemistry pro!
Here’s the deal: each compound has two parts. The first part of the name tells you the first element, and the second part tells you the second element with an “-ide” ending. For example, in carbon dioxide (CO₂), “carbon” is the first element, and “dioxide” tells you there are two oxygen atoms.
To make things clearer, we use prefixes to show how many atoms of each element are in the compound. These prefixes are: mono- (1), di- (2), tri- (3), tetra- (4), penta- (5), hexa- (6), and so on. But here’s a tip: if there’s only one atom of the first element, we usually skip the “mono-.” So, CO is carbon monoxide, not monocarbon monoxide.
Let’s break down another example: dinitrogen tetroxide (N₂O₄). “Di-” means two nitrogen atoms, and “tetra-” means four oxygen atoms.
Naming molecular compounds might seem like cracking a code, but once you know the rules, it’s a piece of cake. You’ll be able to decipher and create the names of compounds like a chemistry pro!
Комментарии
 0:10:11
0:10:11
 0:10:46
0:10:46
 0:10:32
0:10:32
 0:04:17
0:04:17
 0:05:44
0:05:44
 0:13:33
0:13:33
 0:05:35
0:05:35
 0:10:43
0:10:43
 1:32:19
1:32:19
 0:04:43
0:04:43
 0:04:42
0:04:42
 0:08:44
0:08:44
 0:07:39
0:07:39
 0:05:17
0:05:17
 0:08:00
0:08:00
 0:05:01
0:05:01
 0:10:00
0:10:00
 0:10:22
0:10:22
 0:09:31
0:09:31
 0:03:55
0:03:55
 0:03:27
0:03:27
 0:07:32
0:07:32
 0:02:35
0:02:35
 0:11:11
0:11:11