filmov
tv
JEE Advanced Physics 2021 Shift 1 #2 Thermodynamic Cyclic Process

Показать описание
To donate:
An ideal gas undergoes a four step cycle as shown in the P-V diagram below. During this cycle heat is absorbed by the as in:
A) steps 1 and 2
B) steps 1 and 3
C) steps 1 and 4
D) steps 2 and 4
Previous video in this series can be seen at:
Next video in this series can be seen at:
JEE Advanced Physics 2021 Shift 1 #13 Torque and Angular Momentum
JEE Advanced 2021 solutions Physics Paper 1
JEE Advanced 2021 solutions Physics Paper 2
JEE Advanced 2021 Paper Analysis
7 Most Important Chapters in JEE Advanced 2023 Physics | JEE Physics #shorts #iit #jee #esaral
Oxford student reacts to India’s JEE Advanced exam paper *really hard* #shorts #viral #jeeadvanced
Checking Jee main result 2022//AIR 3
😍I proposed her! IIT Placement in 2023 | IIT Motivation | JEE 2023 | JEE 2024 #iit #jee
JEE Advanced 2021 Question Paper Solutions with Answer Key for JEE 2023 Aspirants!
Toughest question of IIT JEE Advanced ever 😱
JEE Advanced 2021 [ Maths & Physics With Answer Key & Analysis] Part-1 | Vedantu JEE Enthuse...
Checking My Jee Advanced Result 2023 Live | My Jee advanced Rank? | Jee Advanced 2023
When an IITian returns to Home❤️💯💪🏻🔥IIT Bombay #ytshorts#trending#shorts#jee2023#vlog#iit#mothersday...
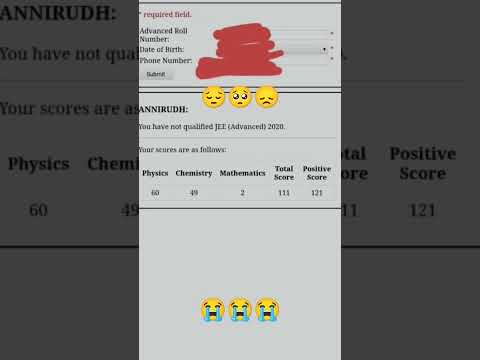
😭A student who didn't qualify jee advanced| subject wise cutoff not clear | #iit #jeemains #jee...
Psychology Behind Cracking JEE Advanced | JEE Advanced 2021 Strategy | Physics Wallah #Shorts
My Transformation || Before and After Jee #iit #viral #shorts #study #jee #motivation #trending
JEE Advanced 2021 [ Question Paper Solutions With Answer Key ] | Vedantu JEE✌
JEE Advanced 2021 Paper - 1 Discussion | JEE Advanced 2021 Paper -1 Solutions | Unacademy JEE
JEE Advanced 2021 Question Paper Analysis (1 & 2) | Maths, Physics, Chemistry Questions
Percentile Vs College || JEE Mains 2024
Solving IIT-JEE question in 30 seconds!!
Best Of JEE Advanced Previous Year Questions [JEE Advanced 2021] | Mission IIT🎯 | JEE English
🤯My IIT JEE story in 30 seconds | Motivational Story | JEE Mains 2022 | IIT Motivation | #iit #jee...
❤️ IIT Bombay Classrooms Actual View 🥰 IIT Bombay Motivation🔥 #iitbombay #motivation #shorts...
Комментарии
 0:06:44
0:06:44
 0:48:22
0:48:22
 0:48:12
0:48:12
 0:04:57
0:04:57
 0:00:40
0:00:40
 0:01:00
0:01:00
 0:00:29
0:00:29
 0:00:32
0:00:32
 4:21:37
4:21:37
 0:01:00
0:01:00
 3:20:50
3:20:50
 0:00:50
0:00:50
 0:00:45
0:00:45
 0:00:13
0:00:13
 0:01:00
0:01:00
 0:00:16
0:00:16
 8:57:41
8:57:41
 6:15:42
6:15:42
 0:29:55
0:29:55
 0:01:00
0:01:00
 0:00:29
0:00:29
 1:20:39
1:20:39
 0:00:30
0:00:30
 0:00:40
0:00:40