filmov
tv
Molar Mass / Molecular Weight of FeSO4: Iron (II) sulfate

Показать описание
Explanation of how to find the molar mass of FeSO4: Iron (II) sulfate.
A few things to consider when finding the molar mass for FeSO4:
- make sure you have the correct chemical formula.
- always include the units for molecular weight (grams/mole).
- make sure you do the math right - follow the order of operations.
Note that molecular weight, molar mass, and gram formula mass are all the same thing.
Finding the Molar Mass (sometimes called Molecular Weight although the units are different) of a compound is a essential skill for the chemistry topic of stoichiometry and the first step in converting from moles to grams (or grams to moles).
A few things to consider when finding the molar mass for FeSO4:
- make sure you have the correct chemical formula.
- always include the units for molecular weight (grams/mole).
- make sure you do the math right - follow the order of operations.
Note that molecular weight, molar mass, and gram formula mass are all the same thing.
Finding the Molar Mass (sometimes called Molecular Weight although the units are different) of a compound is a essential skill for the chemistry topic of stoichiometry and the first step in converting from moles to grams (or grams to moles).
How to Calculate Molar Mass (Molecular Weight)
How To Calculate The Molar Mass of a Compound - Quick & Easy!
How To Calculate Molecular Weight and Molar Mass!
Molar Mass/Molecular Weight Calculation with Parentheses (Example)
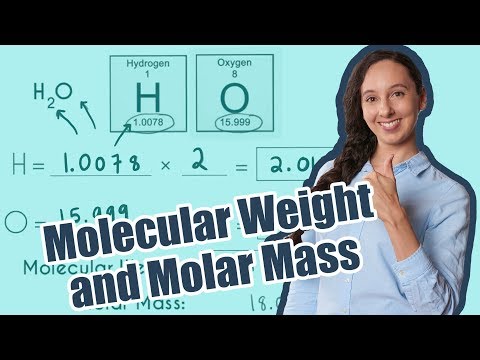
Molar Mass / Molecular Weight of H2O: Water
How to Calculate Molar Mass Practice Problems
Molar Mass / Molecular Weight of O2 (Oxygen Gas)
Molar Mass / Molecular Weight of H2: Hydrogen Gas
Solutions and Colligative Properties #class12 #chemistry #saitechinfo
How to Calculate the Molar Mass / Molecular Weight of Fe2O3 --- Iron (III) Oxide
Molar Mass / Molecular Weight of NO3-
NaCl Molar Mass / Molecular Weight
Molar Mass / Molecular Weight of N2: Nitrogen Gas
Molar Mass / Molecular Weight of HCl : Hydrochloric acid
Molar Mass / Molecular Weight of C6H12O6 (Glucose)
Molar Mass / Molecular Weight of CH4 (Methane)
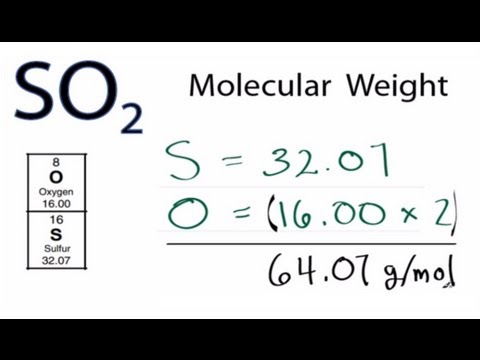
Molar Mass / Molecular Weight of SO2: Sulfur dioxide
Determining Molar Mass (Gram Formula Mass, Molecular Mass, Molecular Weight...)
Molar Mass / Molecular Weight of CuSO4: Copper (II) sulfate
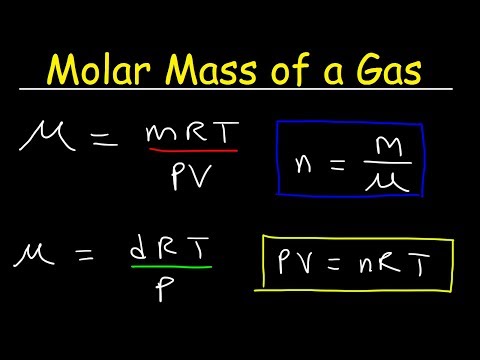
Molar Mass of a Gas at STP - Equations & Formulas, Chemistry Practice Problems
calculation of molar mass|chemistry world |
Molar Mass / Molecular Weight of Al2O3 (Aluminum oxide)
Molar Mass / Molecular Weight of KMnO4 (Potassium Permanganate)
Molar Mass / Molecular Weight of H2SO4: Sulfuric acid
Комментарии
 0:03:51
0:03:51
 0:11:20
0:11:20
 0:06:03
0:06:03
 0:01:45
0:01:45
 0:00:56
0:00:56
 0:13:11
0:13:11
 0:01:09
0:01:09
 0:00:41
0:00:41
 3:07:35
3:07:35
 0:01:37
0:01:37
 0:00:44
0:00:44
 0:00:58
0:00:58
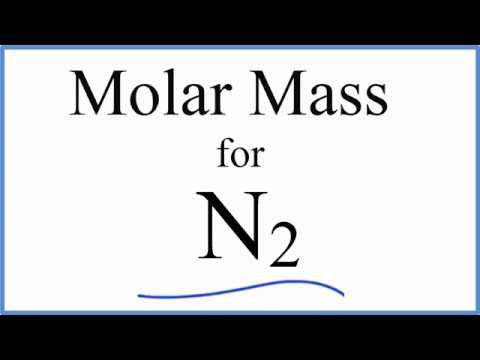 0:00:41
0:00:41
 0:00:34
0:00:34
 0:01:16
0:01:16
 0:00:52
0:00:52
 0:00:44
0:00:44
 0:04:10
0:04:10
 0:01:31
0:01:31
 0:10:41
0:10:41
 0:00:06
0:00:06
 0:01:19
0:01:19
 0:01:11
0:01:11
 0:01:15
0:01:15