filmov
tv
The empirical formula and molecular mass of a compound are CH2O and 180g respectively. What will...

Показать описание
NCERT Exemplar Page No. 3 Some Basic Concepts of Chemistry
Problem 10:- The empirical formula and molecular mass of a compound are CH2O and 180g respectively. What will be the molecular formula of the compound?
(i) C9H18O9 (ii) CH2O (iii) C6H12O6 (iv) C2H4O2
Problem 10:- The empirical formula and molecular mass of a compound are CH2O and 180g respectively. What will be the molecular formula of the compound?
(i) C9H18O9 (ii) CH2O (iii) C6H12O6 (iv) C2H4O2
Empirical Formula & Molecular Formula Determination From Percent Composition
Empirical Formula and Molecular Formula Introduction
Empirical Formula vs Molecular Formula (Definitions & Examples)
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
Calculating Molecular Formula from Empirical Formula
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Practice Problem: Empirical and Molecular Formulas
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
Empirical, molecular, and structural formulas | AP Chemistry | Khan Academy
How to calculate Empirical Formula? 3 Easy Steps
Writing Empirical Formula Practice Problems
Empirical and Molecular formula: Chemistry | Stoichiometry
Empirical and molecular formula grade 11
Molecular and empirical formula
Empirical Formulae From Percentage Composition | Chemical Calculations | Chemistry | FuseSchool
Calculating Molecular Formulas Step by Step | How to Pass Chemistry
Determining Empirical and Molecular Formulas - Chemistry Tutorial
Empirical and Molecular Formula from Percent Composition (No. 1)
Emperical and Molecular Formula 2 (Chemistry JAMB and PUTME Class)
Emperical and Molecular Formula 1 (Chemistry JAMB and PUTME Class)
From the Molecular Formula to the Empirical Formula
The difference between molecular and empirical formula
Empirical Formula & Molecular Formula & Water of Crystallisation - A level Chemistry
Empirical Formula: How to calculate | Stoichiometry | Chemistry
Комментарии
 0:11:00
0:11:00
 0:08:31
0:08:31
 0:03:19
0:03:19
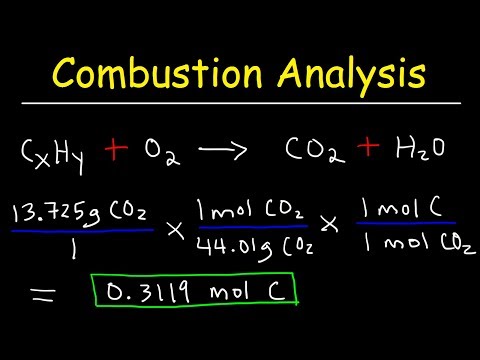 0:16:49
0:16:49
 0:09:09
0:09:09
 0:13:30
0:13:30
 0:05:53
0:05:53
 0:02:52
0:02:52
 0:06:49
0:06:49
 0:05:01
0:05:01
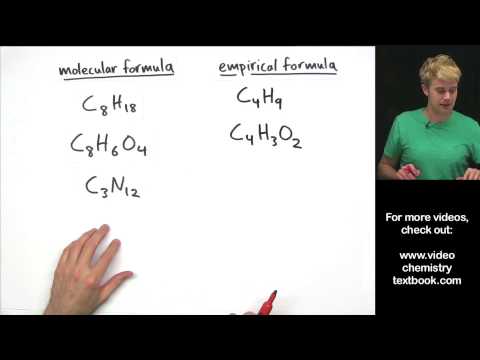 0:06:09
0:06:09
 0:02:52
0:02:52
 0:13:01
0:13:01
 0:03:42
0:03:42
 0:04:33
0:04:33
 0:04:26
0:04:26
 0:08:10
0:08:10
 0:08:47
0:08:47
 0:16:56
0:16:56
 0:31:16
0:31:16
 0:05:52
0:05:52
 0:02:51
0:02:51
 0:22:51
0:22:51
 0:08:50
0:08:50