filmov
tv
Cannizzaro's reaction Mechanism || With easy tricks || By MA Chemistry
Показать описание
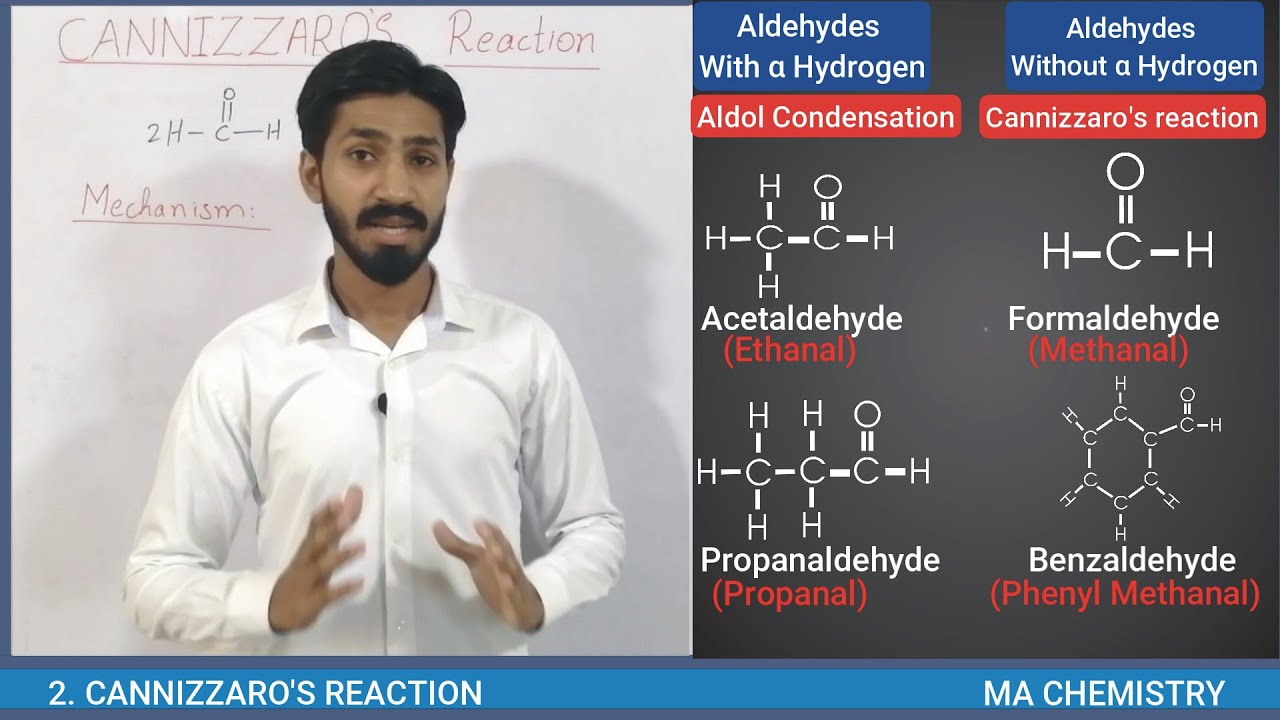
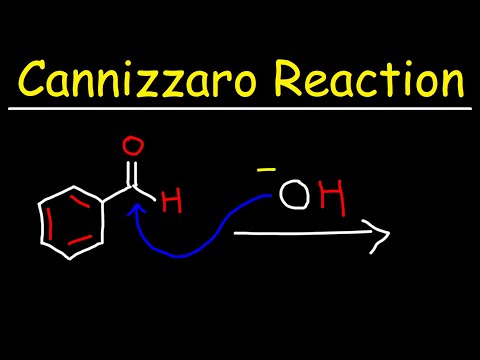
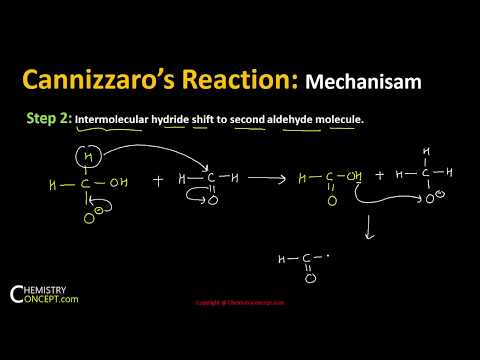
Cannizzaro Reaction Mechanism details the method to get one molecule of alcohol and one molecule of carboxylic acid from two molecules of a given aldehyde. Scientist Stanislao Cannizzaro, in 1853 succeeded in obtaining benzyl alcohol and potassium benzoate from benzaldehyde. The reaction is executed by a nucleophilic acyl substitution on an aldehyde where the leaving group attacks another aldehyde. A tetrahedral intermediate results from the attack of hydroxide on a carbonyl. This tetrahedral intermediate collapses, thereby reforming the carbonyl and transferring a hydride which attacks another colony.
Now, a proton is exchanged by acid and alkoxide ions. When a base of high concentration is introduced, the aldehyde forms an anion which has a charge of 2. From this, a hydride ion is transferred to a second molecule of the aldehyde, forming carboxylate and alkoxide ions. The alkoxide ion also obtains a proton from the solvent for the reaction.
Now, a proton is exchanged by acid and alkoxide ions. When a base of high concentration is introduced, the aldehyde forms an anion which has a charge of 2. From this, a hydride ion is transferred to a second molecule of the aldehyde, forming carboxylate and alkoxide ions. The alkoxide ion also obtains a proton from the solvent for the reaction.
Cannizzaro Reaction Mechanism
Cannizzaro Reaction Mechanism Part-1 Tutorial by C.V. Kalyan Kumar
Cannizzaro Reaction | Mechanism of Cannizzaro Reaction
Cannizzaro Reaction
Cannizzaro reaction | Reactions and mechanism of Cannizzaro reaction | 12th class chemistry | ch#12
The Cannizzaro reaction
Cannizzaro Reaction Mechanism | Inter- and intramolecular Cannizzaro reaction| Cross Cannizzaro |
Ch#19 |Lec#5 | Cannizzaro Reactions, Disproportionation reaction, Mechanism, Class 12,organic
Aldehydes, Ketones & Carboxylic Acids Lecture-10 || Class-12th CBSE/BSEB || Exam Special Series
Cannizzaro Reaction and Mechanism | Name Reactions
Cannizzaro reaction and cross Cannizzaro reaction class 12 CBSE chemistry english
🔥 Cannizzaro reaction Funny Trick in Organic chemistry | Vineet khatri
Cannizzaro reaction Super Trick Organic Chemistry | IIT JEE & NEET | ATP STAR Kota | Vineet khat...
Cannizzaro Reaction and Mechanism ( Dixit Sir )
CANNIZZARO REACTION | MECHANISM
Cannizzaro Reaction Mechanism Part 1 | Coffee with Chemistry | Cannizzaro Reaction in Hindi | Ep 11
Cannizzaro Reaction Trick | Organic Chemistry #neet #jee
Cannizzaro Reaction (organic named reactions) class 12 organic chemistry
Cannizzaro Reaction : Benzyl alcohol and Benzoic Acid from Benzaldehyde
Cannizzaro Reaction (Part 2): Examples and Application.
Cannizzaro Reaction with mechanism, limitation and Applications | Dr. Bharat Baria
Cannizzaro Reaction | Everything You Need to Know | Jee Adavnced | NEET | AIIMS | Mains
Cannizzaro Reaction: Theory
Cannizzaro Reaction with mechanism ( from basics) - for JEE/NEET & Basic learners
Комментарии
 0:05:28
0:05:28
 0:07:08
0:07:08
 0:09:48
0:09:48
 0:09:11
0:09:11
 0:20:22
0:20:22
 0:20:43
0:20:43
 0:23:10
0:23:10
 0:19:20
0:19:20
 0:56:35
0:56:35
 0:06:04
0:06:04
 0:12:32
0:12:32
 0:00:45
0:00:45
 0:03:48
0:03:48
 0:15:45
0:15:45
 0:07:07
0:07:07
 0:22:35
0:22:35
 0:00:44
0:00:44
 0:06:22
0:06:22
 0:08:33
0:08:33
 0:23:50
0:23:50
 0:43:10
0:43:10
 0:43:16
0:43:16
 0:01:49
0:01:49
 0:17:34
0:17:34