filmov
tv
R3.2.14 Cell potential and Gibbs free energy (HL)

Показать описание
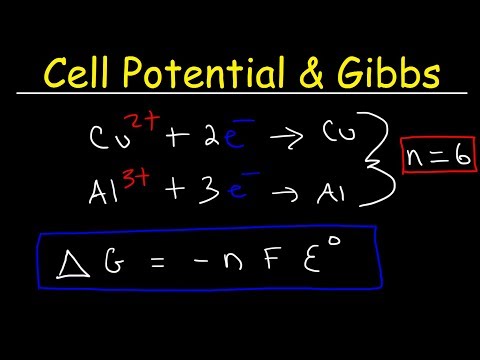
This video covers how to calculate Gibbs's energy from Ecell.
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
18.7 Cell Potential and Free Energy in Galvanic Cells
Cell potential and Gibbs energy
19.1 Cell potential and Gibbs free energy (HL)
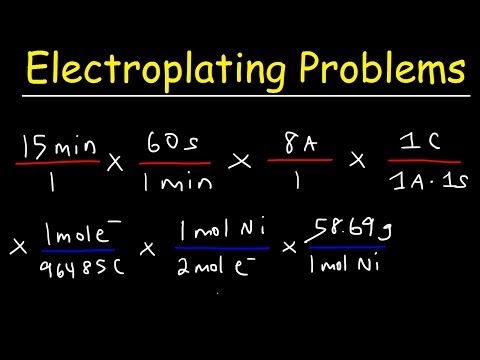
Introduction to Electroplating - Electrochemistry
19.4 How to Calculate Standard Cell Potential | General Chemistry
IB Chemistry Topic 9.2 & 19.1 PART 6: Calculate Voltaic Cell Electrode Potential & Spontanei...
terms, definitions & Units used in Electrochemistry
electcrochemical cell potential
Calculating Cell Potential
IB Chemistry Topic 9 Redox processes Topic 19.1 Electrochemical cells HL
Nernst Equation For electrode potential ,Gibbs free energy & E°cell||Class12 ||Chemistry ||U-3||...
9. Galvanic cell potential calculations (HSC chemistry)
R2.3.7Delta G Theta = -RTlnK (Gibbs/Equilibrium Constant calculations) [HL IB Chemistry]
'Cell Potential & Concentration' | AP Chemistry with Educator.com
Electrochemistry: A Disproportionation Reaction
Electrolysis & Electroplating Practice Problems - Electrochemistry
Lecture 2 Equilibrium at the interface
How to convert the spontaneous to non-spontaneous reaction-Part-2: Nernst/Over Potential, IR drop
[H2 Chemistry] 2022 Topic 20 Electrochemistry 2
Calculate the potential for half cell containing: \( 0.10 \mathrm{M} \mathrm{K}_{2} \mathrm{Cr}_...
16.04 Standard Reduction Potentials
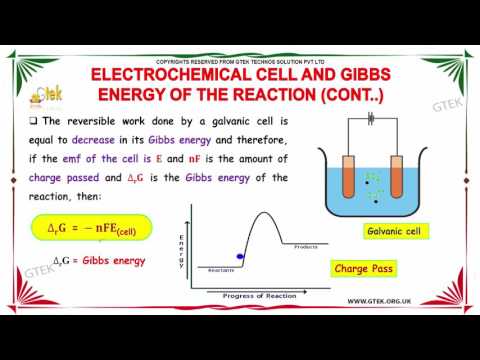
electrochemical cell and gibbs free energy reaction electrochemistry 2 12 chemistry subject cbse
WCLN - Electrochemical Cells-Finding Initial Voltage - Chemistry
Комментарии
 0:11:02
0:11:02
 0:11:25
0:11:25
 0:11:01
0:11:01
 0:02:16
0:02:16
 0:07:05
0:07:05
 0:27:14
0:27:14
 0:15:00
0:15:00
 0:10:27
0:10:27
 0:11:39
0:11:39
 0:05:48
0:05:48
 0:16:28
0:16:28
 0:34:16
0:34:16
 0:14:46
0:14:46
 0:04:53
0:04:53
 0:05:31
0:05:31
 0:06:59
0:06:59
 0:20:47
0:20:47
 0:06:33
0:06:33
 0:30:51
0:30:51
![[H2 Chemistry] 2022](https://i.ytimg.com/vi/TKydG5DSz1M/hqdefault.jpg) 1:31:09
1:31:09
 0:09:16
0:09:16
 0:12:14
0:12:14
 0:02:29
0:02:29
 0:09:21
0:09:21